SNAKEBITE ENVENOMATION and DEATH in the DEVELOPING WORLD Luzia S. Cruz, MD
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Snake Bite Protocol
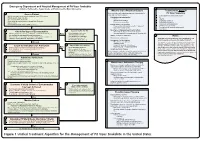
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Major Article Epidemiological Study of Snakebite Cases in Brazilian Western Amazonia
Rev Soc Bras Med Trop 51(3):338-346, May-June, 2018 doi: 10.1590/0037-8682-0489-2017 Major Article Epidemiological study of snakebite cases in Brazilian Western Amazonia Katia Regina Pena Schesquini Roriz[1], Kayena Delaix Zaqueo[2],[3], Sulamita Silva Setubal[2], Tony Hiroshi Katsuragawa[4], Renato Roriz da Silva[1], Carla Freire Celedônio Fernandes[2],[4], Luiz Augusto Paiva Cardoso[5], Moreno Magalhães de Souza Rodrigues[2], Andreimar Martins Soares[2], Rodrigo Guerino Stábeli[1],[2] and Juliana Pavan Zuliani[1],[2] [1]. Departamento de Medicina, Universidade Federal de Rondônia, Porto Velho, RO, Brasil. [2]. Fundação Oswaldo Cruz-Rondônia, Porto Velho, RO, Brasil. [3]. Centro de Referência de Jaciara, Instituto Federal de Educação, Ciência e Tecnologia de Mato Grosso, Campus São Vicente, Jaciara, MT, Brasil. [4]. Centro de Pesquisa em Medicina Tropical, Porto Velho, RO, Brasil. [5]. Centro de Medicina Tropical de Rondônia, Porto Velho, RO, Brasil. Abstract Introduction: Brazil has the largest number of snakebite cases in South America, of which the large majority is concentrated in the Midwest and North. Methods: In this descriptive observational study, we assessed the epidemiological and clinical snakebite cases referred to the Centro de Medicina Tropical de Rondônia from September 2008 to September 2010. Results: We followed up 92 cases from admission until discharge, namely 81 (88%) men and 11 (12%) women, with a mean age of 37 years, and mainly from rural areas (91.3%). The snakebites occurred while performing work activities (63%) during the Amazon rainy season (78.3%). The vast majority of individuals presented from the Porto Velho microregion (84.7%). -

Cohabitation by Bothrops Asper (Garman 1883) and Leptodactylus Savagei (Heyer 2005)
Herpetology Notes, volume 12: 969-970 (2019) (published online on 10 October 2019) Cohabitation by Bothrops asper (Garman 1883) and Leptodactylus savagei (Heyer 2005) Todd R. Lewis1 and Rowland Griffin2 Bothrops asper is one of the largest (up to 245 cm) log-pile habitat (approximately 50 x 70 x 100cm) during pit vipers in Central America (Hardy, 1994; Rojas day and night. Two adults (with distinguishable size et al., 1997; Campbell and Lamar, 2004). Its range and markings) appeared resident with multiple counts extends from northern Mexico to the Pacific Lowlands (>20). Adults of B. asper were identified individually of Ecuador. In Costa Rica it is found predominantly in by approximate size, markings, and position on the log- Atlantic Lowland Wet forests. Leptodactylus savagei, pile. The above two adults were encountered on multiple a large (up to 180 mm females: 170 mm males snout- occasions between November 2002 and December vent length [SVL]), nocturnal, ground-dwelling anuran, 2003 and both used the same single escape hole when is found in both Pacific and Atlantic rainforests from disturbed during the day. Honduras into Colombia (Heyer, 2005). Across their On 20 November 2002, two nights after first locating ranges, both species probably originated from old forest and observing the above two Bothrops asper, a large but now are also found in secondary forest, agricultural, (131mm SVL) adult Leptodactylus savagei was seen disturbed and human inhabited land (McCranie and less than 2m from two coiled pit vipers (23:00 PM local Wilson, 2002; Savage, 2002; Sasa et al., 2009). Such time). When disturbed, it retreated into the same hole the habitat adaptation is most likely aided by tolerance for a adult pit vipers previously escaped to in the daytime. -

Approach and Management of Spider Bites for the Primary Care Physician
Osteopathic Family Physician (2011) 3, 149-153 Approach and management of spider bites for the primary care physician John Ashurst, DO,a Joe Sexton, MD,a Matt Cook, DOb From the Departments of aEmergency Medicine and bToxicology, Lehigh Valley Health Network, Allentown, PA. KEYWORDS: Summary The class Arachnida of the phylum Arthropoda comprises an estimated 100,000 species Spider bite; worldwide. However, only a handful of these species can cause clinical effects in humans because many Black widow; are unable to penetrate the skin, whereas others only inject prey-specific venom. The bite from a widow Brown recluse spider will produce local symptoms that include muscle spasm and systemic symptoms that resemble acute abdomen. The bite from a brown recluse locally will resemble a target lesion but will develop into an ulcerative, necrotic lesion over time. Spider bites can be prevented by several simple measures including home cleanliness and wearing the proper attire while working outdoors. Although most spider bites cause only local tissue swelling, early species identification coupled with species-specific man- agement may decrease the rate of morbidity associated with bites. © 2011 Elsevier Inc. All rights reserved. More than 100,000 species of spiders are found world- homes and yards in the southwestern United States, and wide. Persons seeking medical attention as a result of spider related species occur in the temperate climate zones across bites is estimated at 50,000 patients per year.1,2 Although the globe.2,5 The second, Lactrodectus, or the common almost all species of spiders possess some level of venom, widow spider, are found in both temperate and tropical 2 the majority are considered harmless to humans. -

Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation
toxins Article Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation Leijiane F. Sousa 1,2, Christina N. Zdenek 2 , James S. Dobson 2, Bianca op den Brouw 2 , Francisco Coimbra 2, Amber Gillett 3, Tiago H. M. Del-Rei 1, Hipócrates de M. Chalkidis 4, Sávio Sant’Anna 5, Marisa M. Teixeira-da-Rocha 5, Kathleen Grego 5, Silvia R. Travaglia Cardoso 6 , Ana M. Moura da Silva 1 and Bryan G. Fry 2,* 1 Laboratório de Imunopatologia, Instituto Butantan, São Paulo 05503-900, Brazil; [email protected] (L.F.S.); [email protected] (T.H.M.D.-R.); [email protected] (A.M.M.d.S.) 2 Venom Evolution Lab, School of Biological Sciences, University of Queensland, St. Lucia, QLD 4072, Australia; [email protected] (C.N.Z.); [email protected] (J.S.D.); [email protected] (B.o.d.B.); [email protected] (F.C.) 3 Fauna Vet Wildlife Consultancy, Glass House Mountains, QLD 4518, Australia; [email protected] 4 Laboratório de Pesquisas Zoológicas, Unama Centro Universitário da Amazônia, Pará 68035-110, Brazil; [email protected] 5 Laboratório de Herpetologia, Instituto Butantan, São Paulo 05503-900, Brazil; [email protected] (S.S.); [email protected] (M.M.T.-d.-R.); [email protected] (K.G.) 6 Museu Biológico, Insituto Butantan, São Paulo 05503-900, Brazil; [email protected] * Correspondence: [email protected] Received: 18 September 2018; Accepted: 8 October 2018; Published: 11 October 2018 Abstract: Lancehead pit-vipers (Bothrops genus) are an extremely diverse and medically important group responsible for the greatest number of snakebite envenomations and deaths in South America. -

Long-Term Effects of Snake Envenoming
toxins Review Long-Term Effects of Snake Envenoming Subodha Waiddyanatha 1,2, Anjana Silva 1,2 , Sisira Siribaddana 1 and Geoffrey K. Isbister 2,3,* 1 Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Saliyapura 50008, Sri Lanka; [email protected] (S.W.); [email protected] (A.S.); [email protected] (S.S.) 2 South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, University of Peradeniya, Peradeniya 20400, Sri Lanka 3 Clinical Toxicology Research Group, University of Newcastle, Callaghan, NSW 2308, Australia * Correspondence: [email protected] or [email protected]; Tel.: +612-4921-1211 Received: 14 March 2019; Accepted: 29 March 2019; Published: 31 March 2019 Abstract: Long-term effects of envenoming compromise the quality of life of the survivors of snakebite. We searched MEDLINE (from 1946) and EMBASE (from 1947) until October 2018 for clinical literature on the long-term effects of snake envenoming using different combinations of search terms. We classified conditions that last or appear more than six weeks following envenoming as long term or delayed effects of envenoming. Of 257 records identified, 51 articles describe the long-term effects of snake envenoming and were reviewed. Disability due to amputations, deformities, contracture formation, and chronic ulceration, rarely with malignant change, have resulted from local necrosis due to bites mainly from African and Asian cobras, and Central and South American Pit-vipers. Progression of acute kidney injury into chronic renal failure in Russell’s viper bites has been reported in several studies from India and Sri Lanka. Neuromuscular toxicity does not appear to result in long-term effects. -

Venom Evolution Widespread in Fishes: a Phylogenetic Road Map for the Bioprospecting of Piscine Venoms
Journal of Heredity 2006:97(3):206–217 ª The American Genetic Association. 2006. All rights reserved. doi:10.1093/jhered/esj034 For permissions, please email: [email protected]. Advance Access publication June 1, 2006 Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms WILLIAM LEO SMITH AND WARD C. WHEELER From the Department of Ecology, Evolution, and Environmental Biology, Columbia University, 1200 Amsterdam Avenue, New York, NY 10027 (Leo Smith); Division of Vertebrate Zoology (Ichthyology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Leo Smith); and Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Wheeler). Address correspondence to W. L. Smith at the address above, or e-mail: [email protected]. Abstract Knowledge of evolutionary relationships or phylogeny allows for effective predictions about the unstudied characteristics of species. These include the presence and biological activity of an organism’s venoms. To date, most venom bioprospecting has focused on snakes, resulting in six stroke and cancer treatment drugs that are nearing U.S. Food and Drug Administration review. Fishes, however, with thousands of venoms, represent an untapped resource of natural products. The first step in- volved in the efficient bioprospecting of these compounds is a phylogeny of venomous fishes. Here, we show the results of such an analysis and provide the first explicit suborder-level phylogeny for spiny-rayed fishes. The results, based on ;1.1 million aligned base pairs, suggest that, in contrast to previous estimates of 200 venomous fishes, .1,200 fishes in 12 clades should be presumed venomous. -

Reptiles and Amphibians of Lamanai Outpost Lodge, Belize
Reptiles and Amphibians of the Lamanai Outpost Lodge, Orange Walk District, Belize Ryan L. Lynch, Mike Rochford, Laura A. Brandt and Frank J. Mazzotti University of Florida, Fort Lauderdale Research and Education Center; 3205 College Avenue; Fort Lauderdale, Florida 33314 All pictures taken by RLL: [email protected] and MR: [email protected] Vaillant’s Frog Rio Grande Leopard Frog Common Mexican Treefrog Rana vaillanti Rana berlandieri Smilisca baudinii Veined Treefrog Red Eyed Treefrog Stauffer’s Treefrog Phrynohyas venulosa Agalychnis callidryas Scinax staufferi White-lipped Frog Fringe-toed Foam Frog Fringe-toed Foam Frog Leptodactylus labialis Leptodactylus melanonotus Leptodactylus melanonotus 1 Reptiles and Amphibians of the Lamanai Outpost Lodge, Orange Walk District, Belize Ryan L. Lynch, Mike Rochford, Laura A. Brandt and Frank J. Mazzotti University of Florida, Fort Lauderdale Research and Education Center; 3205 College Avenue; Fort Lauderdale, Florida 33314 All pictures taken by RLL: [email protected] and MR: [email protected] Tungara Frog Marine Toad Gulf Coast Toad Physalaemus pustulosus Bufo marinus Bufo valliceps Sheep Toad House Gecko Dwarf Bark Gecko Hypopachus variolosus Hemidactylus frenatus Shaerodactylus millepunctatus Turnip-tailed Gecko Yucatan Banded Gecko Yucatan Banded Gecko Thecadactylus rapicaudus Coleonyx elegans Coleonyx elegans 2 Reptiles and Amphibians of the Lamanai Outpost Lodge, Orange Walk District, Belize Ryan L. Lynch, Mike Rochford, Laura A. Brandt and Frank J. Mazzotti University -

The Venomous Snakes of Texas Health Service Region 6/5S
The Venomous Snakes of Texas Health Service Region 6/5S: A Reference to Snake Identification, Field Safety, Basic Safe Capture and Handling Methods and First Aid Measures for Reptile Envenomation Edward J. Wozniak DVM, PhD, William M. Niederhofer ACO & John Wisser MS. Texas A&M University Health Science Center, Institute for Biosciences and Technology, Program for Animal Resources, 2121 W Holcombe Blvd, Houston, TX 77030 (Wozniak) City Of Pearland Animal Control, 2002 Old Alvin Rd. Pearland, Texas 77581 (Niederhofer) 464 County Road 949 E Alvin, Texas 77511 (Wisser) Corresponding Author: Edward J. Wozniak DVM, PhD, Texas A&M University Health Science Center, Institute for Biosciences and Technology, Program for Animal Resources, 2121 W Holcombe Blvd, Houston, TX 77030 [email protected] ABSTRACT: Each year numerous emergency response personnel including animal control officers, police officers, wildlife rehabilitators, public health officers and others either respond to calls involving venomous snakes or are forced to venture into the haunts of these animals in the scope of their regular duties. North America is home to two distinct families of native venomous snakes: Viperidae (rattlesnakes, copperheads and cottonmouths) and Elapidae (coral snakes) and southeastern Texas has indigenous species representing both groups. While some of these snakes are easily identified, some are not and many rank amongst the most feared and misunderstood animals on earth. This article specifically addresses all of the native species of venomous snakes that inhabit Health Service Region 6/5s and is intended to serve as a reference to snake identification, field safety, basic safe capture and handling methods and the currently recommended first aide measures for reptile envenomation. -

Rattlesnake Envenomation
Rattlesnake Bite Reviewers: Shawn M. Varney, Authors: Laura Morrison, MD / Ryan Chuang, MD MD Target Audience: Emergency Medicine Residents (junior and senior level postgraduate learners), Medical Students Primary Learning Objectives: 1. Recognize signs and symptoms of a venomous snakebite. 2. Recognize the indications for antivenom administration. 3. Recognize potentially dangerous complications of a snakebite and antivenom administration. Secondary Learning Objectives: detailed technical/behavioral goals, didactic points 1. Obtain medication history and evaluate for use of anticoagulant medications 2. Discuss importance of reevaluation of local effects 3. Describe the importance of removing tourniquets that may have been placed prior to arrival in ED 4. Describe the types of toxicity that can result from rattlesnake envenomation Critical actions checklist: 1. Obtain peripheral IV access (in contralateral arm) 2. Order coagulation studies (CBC, platelets, INR, fibrinogen) 3. Administer antivenom 4. Stop antivenom infusion when anaphylactic/anaphylactoid reaction to antivenom develops 5. Provide volume resuscitation for anaphylactic/anaphylactoid reaction 6. Administer medications for anaphylaxis 7. Consult Poison Center/Toxicologist 8. Admit to the MICU Environment: Emergency Department treatment area 1. Room Set Up – ED critical care area a. Manikin Set Up – Mid or high fidelity simulator b. Props – Standard ED equipment 2. Distractors – ED noise, alarming monitor For Examiner Only CASE SUMMARY SYNOPSIS OF HISTORY/ Scenario Background This is a case of a 32-year-old man who is brought to the emergency department reporting a snakebite by a prairie rattlesnake while working in the local zoo that afternoon. He reports severe pain at the site and progressive swelling up his arm since the bite 2-3 hours ago. -

EMS DIVISION 24.1 Rev. 05/14/2021 PROTOCOL 24 ENVENOMATION, BITES, and STINGS
PROTOCOL 24 ENVENOMATION, BITES, AND STINGS A. North American Pit Vipers B. Coral Snake Bites C. Exotic Snakes D. Brown Recluse Spider Bites E. Black Widow F. Scorpion Stings G. Marine Animal Envenomation H. Marine Animal Stings General Care EMR/BLS 1. Initial Assessment/Care Protocol 1. 2. Attempt to identify the insect, reptile, or animal that caused the injury if it is safe to do so. If unknown or it is a known venomous reptile bite or spider bite, have the FAO contact the Anti-Venom unit. 3. Be alert for the development of any anaphylactic reaction and treat according to the Systemic Reaction Protocol 17. 4. Immobilize the affected area. Keep the patient calm. 5. Remove and secure in a safe location any rings, bracelets, jewelry, etc. that may be in the injured area before swelling becomes too great. 6. Do not apply tourniquets, cold packs, make incisions around the area, or attempt to suction. 7. If unable to contact an Anti-Venom unit, contact the Poison Control Center, 1-800-222-1222 for assistance in managing specific envenomation. Top EMS DIVISION 24.1 Rev. 05/14/2021 PROTOCOL 24 ENVENOMATION, BITES, AND STINGS A. North American Pit Vipers Includes rattlesnakes, copperheads, and cottonmouths. EMR/BLS 1. For any known or suspected bite, refer to Antivenin Bank Procedure 33. Evaluate for specific signs/symptoms: a) Distinct "fang marks" or puncture wounds. b) Swelling and pain at the site. c) Weakness, nausea, and vomiting. d) Paresthesia, fasciculations. e) Numbness and tingling around the face and head. f) Metallic taste, change in taste sensation. -

Neutralizing Capacity of a New Monovalent Anti-Bothrops Atrox Antivenom: Comparison with Two Commercial Antivenoms
BrazilianNeutralizing Journal capacity of Medical of a new and antivenom Biological againstResearch Bothrops (1997) atrox30: 375-379 375 ISSN 0100-879X Neutralizing capacity of a new monovalent anti-Bothrops atrox antivenom: comparison with two commercial antivenoms R. Otero1, V. Núñez1, 1Proyecto de Ofidismo, Facultad de Medicina, J.M. Gutiérrez4, A. Robles4, 2Facultad de Química Farmacéutica, and R. Estrada4, R.G. Osorio2, 3Facultad de Medicina Veterinaria y de Zootecnia, G. Del-Valle3, R. Valderrama1 Universidad de Antioquia, A.A.1226, Medellín, Colombia and C.A. Giraldo1 4Instituto Clodomiro Picado, Facultad de Microbiología, Universidad de Costa Rica, San José, Costa Rica Abstract Correspondence Three horse-derived antivenoms were tested for their ability to neu- Key words R. Otero tralize lethal, hemorrhagic, edema-forming, defibrinating and myotoxic • Bothrops atrox Proyecto de Ofidismo activities induced by the venom of Bothrops atrox from Antioquia and • Snake venom Facultad de Medicina Chocó (Colombia). The following antivenoms were used: a) polyva- • Antivenom Universidad de Antioquia lent (crotaline) antivenom produced by Instituto Clodomiro Picado • Neutralization A.A.1226, Medellín • (Costa Rica), b) monovalent antibothropic antivenom produced by Antioquia Colombia • Chocó Fax: 57-4-263-8282 Instituto Nacional de Salud-INS (Bogotá), and c) a new monovalent anti-B. atrox antivenom produced with the venom of B. atrox from Research supported by the Antioquia and Chocó. The three antivenoms neutralized all toxic Instituto Colombiano para el activities tested albeit with different potencies. The new monovalent Desarrollo de la Ciencia y la anti-B. atrox antivenom showed the highest neutralizing ability against Tecnología Francisco José de edema-forming and defibrinating effects of B.