Snake Venom Detection Kit (SVDK)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurotoxic Effects of Venoms from Seven Species of Australasian Black Snakes (Pseudechis): Efficacy of Black and Tiger Snake Antivenoms
Clinical and Experimental Pharmacology and Physiology (2005) 32, 7–12 NEUROTOXIC EFFECTS OF VENOMS FROM SEVEN SPECIES OF AUSTRALASIAN BLACK SNAKES (PSEUDECHIS): EFFICACY OF BLACK AND TIGER SNAKE ANTIVENOMS Sharmaine Ramasamy,* Bryan G Fry† and Wayne C Hodgson* *Monash Venom Group, Department of Pharmacology, Monash University, Clayton and †Australian Venom Research Unit, Department of Pharmacology, University of Melbourne, Parkville, Victoria, Australia SUMMARY the sole clad of venomous snakes capable of inflicting bites of medical importance in the region.1–3 The Pseudechis genus (black 1. Pseudechis species (black snakes) are among the most snakes) is one of the most widespread, occupying temperate, widespread venomous snakes in Australia. Despite this, very desert and tropical habitats and ranging in size from 1 to 3 m. little is known about the potency of their venoms or the efficacy Pseudechis australis is one of the largest venomous snakes found of the antivenoms used to treat systemic envenomation by these in Australia and is responsible for the vast majority of black snake snakes. The present study investigated the in vitro neurotoxicity envenomations. As such, the venom of P. australis has been the of venoms from seven Australasian Pseudechis species and most extensively studied and is used in the production of black determined the efficacy of black and tiger snake antivenoms snake antivenom. It has been documented that a number of other against this activity. Pseudechis from the Australasian region can cause lethal 2. All venoms (10 g/mL) significantly inhibited indirect envenomation.4 twitches of the chick biventer cervicis nerve–muscle prepar- The envenomation syndrome produced by Pseudechis species ation and responses to exogenous acetylcholine (ACh; varies across the genus and is difficult to characterize because the 1 mmol/L), but not to KCl (40 mmol/L), indicating activity at offending snake is often not identified.3,5 However, symptoms of post-synaptic nicotinic receptors on the skeletal muscle. -
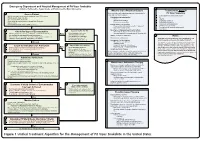
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Letter from the Desk of David Challinor December 1992 We Often
Letter from the Desk of David Challinor December 1992 We often identify poisonous animals as snakes even though no more than a quarter of these reptiles are considered venomous. Snakes have a particular problem that is ameliorated by venom. With nothing to hold its food while eating, a snake can only grab its prey with its open mouth and swallow it whole. Their jaws can unhinge which allows snakes to swallow prey larger in diameter than their own body. Clearly the inside of a snake's mouth and the tract to its stomach must be slippery enough for the prey animal to slide down whole, and saliva provides this lubricant. When food first enters our mouths and we begin to chew, saliva and the enzymes it contains immediately start to break down the material for ease of swallowing. We are seldom aware of our saliva unless our mouths become dry, which triggers us to drink. When confronted with a chocolate sundae or other favorite dessert, humans salivate. The very image of such "mouth watering" food and the anticipation of tasting it causes a reaction in our mouths which prepares us for a delightful experience. Humans are not the only animals that salivate to prepare for eating, and this fluid has achieved some remarkable adaptations in other creatures. scientists believe that snake venom evolved from saliva. Why it became toxic in certain snake species and not in others is unknown, but the ability to produce venom helps snakes capture their prey. A mere glancing bite from a poisonous snake is often adequate to immobilize its quarry. -

Venemous Snakes
WASAH WESTERN AUSTRALIAN SOCIETY of AMATEUR HERPETOLOGISTS (Inc) K E E P I N G A D V I C E S H E E T Venomous Snakes Southern Death Adder (Acanthophis Southern Death antarcticus) – Maximum length 100 cm. Adder Category 5. Desert Death Adder (Acanthophis pyrrhus) – Acanthophis antarcticus Maximum length 75 cm. Category 5. Pilbara Death Adder (Acanthophis wellsi) – Maximum length 70 cm. Category 5. Western Tiger Snake (Notechis scutatus) - Maximum length 160 cm. Category 5. Mulga Snake (Pseudechis australis) – Maximum length 300 cm. Category 5. Spotted Mulga Snake (Pseudechis butleri) – Maximum length 180 cm. Category 5. Dugite (Pseudonaja affinis affinis) – Maximum Desert Death Adder length 180 cm. Category 5. Acanthophis pyrrhus Gwardar (Pseudonaja nuchalis) – Maximum length 100 cm. Category 5. NOTE: All species listed here are dangerously venomous and are listed as Category 5. Only the experienced herpetoculturalist should consider keeping any of them. One must be over 18 years of age to hold a category 5 license. Maintaining a large elapid carries with 1 it a considerable responsibility. Unless you are Pilbara Death Adder confident that you can comply with all your obligations and licence requirements when Acanthophis wellsi keeping dangerous animals, then look to obtaining a non-venomous species instead. NATURAL HABITS: Venomous snakes occur in a wide variety of habitats and, apart from death adders, are highly mobile. All species are active day and night. HOUSING: In all species listed except death adders, one adult (to 150 cm total length) can be kept indoors in a lockable, top-ventilated, all glass or glass-fronted wooden vivarium of Western Tiger Snake at least 90 x 45 cm floor area. -

Effects of Aflatoxins Contaminating Food on Human Health - Magda Carvajal and Pável Castillo
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT - – Vol.VII - Effects of Aflatoxins Contaminating Food on Human Health - Magda Carvajal and Pável Castillo EFFECTS OF AFLATOXINS CONTAMINATING FOOD ON HUMAN HEALTH Magda Carvajal and Pável Castillo Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México. Ciudad Universitaria, Colonia Copilco, Delegación Coyoacán. 04510 México, D.F.(Institute of Biology, National Autonomous University of Mexico). Keywords: Mycotoxins, aflatoxins, cancer, mutagenesis, food contamination, DNA adducts, biomarkers, hepatic diseases, cirrhosis, hepatitis, toxicology, chemical mutations. Contents 1. Aflatoxins, production, occurrence, chemical structure 1.1 Definition of Aflatoxins 1.2. Aflatoxin Producing Fungi and Production Conditions 1.3. Occurrence 1.4. Chemical Structure and Types 1.5. Biological Properties 2. Biosynthetic pathway 2.1. Biotransformation of AFB1 3. Analytical methods for aflatoxin study 4. Aflatoxin metabolism 5. Toxic effects of aflatoxins on animal and human health 5.1 In Plants 5.2. In Animals 5.3. In Humans 6. Economic losses due to aflatoxin contamination 7. Control 7.1. Preventive Measures 7.2. Structural Degradation after Chemical Treatment 7.3. Modification of Toxicity by Dietary Chemicals 7.4. Detoxification 7.5. ChemosorbentsUNESCO – EOLSS 7.6. Radiation 8. Legislation 9. Conclusions Glossary SAMPLE CHAPTERS Bibliography Biographical Sketches Summary Aflatoxins (AF) are toxic metabolites of the moulds Aspergillus flavus, A. parasiticus and A. nomius. AF link to DNA, RNA and proteins and affect all the living kingdom, from viruses to man, causing acute or chronic symptoms, they are mutagens, hepatocarcinogens, and teratogens. ©Encyclopedia of Life Support Systems (EOLSS) TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT - – Vol.VII - Effects of Aflatoxins Contaminating Food on Human Health - Magda Carvajal and Pável Castillo The impact of AF contamination on crops is estimated in hundreds of millions dollars. -

Approach and Management of Spider Bites for the Primary Care Physician
Osteopathic Family Physician (2011) 3, 149-153 Approach and management of spider bites for the primary care physician John Ashurst, DO,a Joe Sexton, MD,a Matt Cook, DOb From the Departments of aEmergency Medicine and bToxicology, Lehigh Valley Health Network, Allentown, PA. KEYWORDS: Summary The class Arachnida of the phylum Arthropoda comprises an estimated 100,000 species Spider bite; worldwide. However, only a handful of these species can cause clinical effects in humans because many Black widow; are unable to penetrate the skin, whereas others only inject prey-specific venom. The bite from a widow Brown recluse spider will produce local symptoms that include muscle spasm and systemic symptoms that resemble acute abdomen. The bite from a brown recluse locally will resemble a target lesion but will develop into an ulcerative, necrotic lesion over time. Spider bites can be prevented by several simple measures including home cleanliness and wearing the proper attire while working outdoors. Although most spider bites cause only local tissue swelling, early species identification coupled with species-specific man- agement may decrease the rate of morbidity associated with bites. © 2011 Elsevier Inc. All rights reserved. More than 100,000 species of spiders are found world- homes and yards in the southwestern United States, and wide. Persons seeking medical attention as a result of spider related species occur in the temperate climate zones across bites is estimated at 50,000 patients per year.1,2 Although the globe.2,5 The second, Lactrodectus, or the common almost all species of spiders possess some level of venom, widow spider, are found in both temperate and tropical 2 the majority are considered harmless to humans. -

Desmodus Rotundus) Blood Feeding
toxins Article Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding Rahini Kakumanu 1, Wayne C. Hodgson 1, Ravina Ravi 1, Alejandro Alagon 2, Richard J. Harris 3 , Andreas Brust 4, Paul F. Alewood 4, Barbara K. Kemp-Harper 1,† and Bryan G. Fry 3,*,† 1 Department of Pharmacology, Biomedicine Discovery Institute, Faculty of Medicine, Nursing & Health Sciences, Monash University, Clayton, Victoria 3800, Australia; [email protected] (R.K.); [email protected] (W.C.H.); [email protected] (R.R.); [email protected] (B.K.K.-H.) 2 Departamento de Medicina Molecular y Bioprocesos, Instituto de Biotecnología, Universidad Nacional Autónoma de México, Av. Universidad 2001, Cuernavaca, Morelos 62210, Mexico; [email protected] 3 Venom Evolution Lab, School of Biological Sciences, University of Queensland, St. Lucia, Queensland 4067, Australia; [email protected] 4 Institute for Molecular Biosciences, University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (A.B.); [email protected] (P.F.A.) * Correspondence: [email protected] † Joint senior authors. Received: 20 November 2018; Accepted: 2 January 2019; Published: 8 January 2019 Abstract: Animals that specialise in blood feeding have particular challenges in obtaining their meal, whereby they impair blood hemostasis by promoting anticoagulation and vasodilation in order to facilitate feeding. These convergent selection pressures have been studied in a number of lineages, ranging from fleas to leeches. However, the vampire bat (Desmondus rotundus) is unstudied in regards to potential vasodilatory mechanisms of their feeding secretions (which are a type of venom). This is despite the intense investigations of their anticoagulant properties which have demonstrated that D. -

Death Adders {Acanthophis Laevis Complex) from the Island of Ambon
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Herpetozoa Jahr/Year: 2006 Band/Volume: 19_1_2 Autor(en)/Author(s): Kuch Ulrich, McGuire Jimmy A., Yuwono Frank Bambang Artikel/Article: Death adders (Acanthophis laevis complex) from the island of Ambon (Maluku, Indonesia) 81-82 ©Österreichische Gesellschaft für Herpetologie e.V., Wien, Austria, download unter www.biologiezentrum.at SHORT NOTE HERPETOZOA 19(1/2) Wien, 30. Juli 2006 SHORT NOTE 81 O. & PINTO, I. & BRUFORD, M. W. & JORDAN, W. C. & NICHOLS, R. A. (2002): The double origin of Iberian peninsular chameleons.- Biological Journal of the Linnean Society, London; 75: 1-7. PINHO, C. & FER- RAND, N. & HARRIS, D. J. (2006): Reexamination of the Iberian and North African Podarcis phylogeny indi- cates unusual relative rates of mitochondrial gene evo- lution in reptiles.- Molecular Phylogenetics and Evolu- tion, Chicago; 38: 266-273. POSADA, D. &. CRANDALL, K. A. (1998): Modeltest: testing the model of DNA substitution- Bioinformatics, Oxford; 14: 817-818. SWOFFORD, D. L. (2002): PAUP*. Phylogenetic analy- sis using parsimony (*and other methods). Version 4.0. Sinauer Associates, Uderland, Massachusetts. WADK, E. (2001): Review of the False Smooth snake genus Macroprotodon (Serpentes, Colubridae) in Algeria with a description of a new species.- Bulletin National Fig. 1 : Adult death adder (Acanthophis laevis com- History Museum London (Zoology), London; 67 (1): plex) from Negeri Lima, Ambon (Central Maluku 85-107. regency, Maluku province, Indonesia). Photograph by U. KUCH. KEYWORDS: mitochondrial DNA, cyto- chrome b, Macroprotodon, evolution, systematics, Iberian Peninsula, North Africa SUBMITTED: April 1,2005 and Bali by the live animal trade. -

Jemena Northern Gas Pipeline Pty Ltd
Jemena Northern Gas Pipeline Pty Ltd Northern Gas Pipeline Draft Environmental Impact Statement CHAPTER 12 – MATTERS OF NATIONAL ENVIRONMENTAL SIGNIFICANCE Public August 2016 MATTERS OF NATIONAL ENVIRONMENTAL SIGNIFICANCE — 12 Contents 12. Matters of National Environmental Significance ............................................................. 12-1 12.1 Overview .................................................................................................................... 12-1 12.2 Relevant MNES ......................................................................................................... 12-2 12.2.1 Threatened species ............................................................................................ 12-2 12.2.2 Plains Death Adder (Acanthophis hawkei) ........................................................ 12-13 12.2.3 Carpentarian Antechinus (Pseudantechinus mimulus) ...................................... 12-18 12.2.4 Threatened species conclusion ......................................................................... 12-23 12.3 Risk assessment ...................................................................................................... 12-23 12.3.1 Potential impacts .............................................................................................. 12-23 12.3.2 Planning ............................................................................................................ 12-24 12.3.3 Construction .................................................................................................... -

Tiger Snake Antivenom
Husbandry Manual for Tiger Snakes Notechis spp (Peters 1861) sl Reptilia:Elapidae Author: John. J. Mostyn Date of Preparation: 2006 Western Sydney Institute of TAFE, Richmond Course Name and Number: Captive Animal Management 1068 Lecturer: Graeme Phipps / Andrew Titmuss/ Jacki Salkeld/ Elissa Smith © 2006 John J Mostyn 1 Occupational Health and Safety WARNING This Snake is DANGEROUSLY VENOMOUS CAPABLE OF INFLICTING A POTENTIALLY FATAL BITE ALWAYS HAVE A COMPRESSION BANDAGE WITHIN REACH FIRST AID FOR A SNAKE BITE 1) Apply a firm, broad, pressure bandage to bitten limb, and if possible, the whole length of limb, firmly. 2) The limb should be immobilized by a splint and kept as still as possible. 3) Keep the patient still and call for ambulance. Immobilization and the use of a pressure bandage reduces the movement of venom from the bite site. This restriction of venom will allow more time to transport the patient to hospital. The patient should remain calm and rest. If possible, transport should be brought to the patient, rather than patient to transport. Fig 1 (Mirtschin, Davis, 1992) 2 Tiger Snake Antivenom What is Tiger Snake Antivenom? Tiger snake antivenom is an injection designed to help neutralize the effect of the poison (venom) of the tiger snake. It is produced by immunizing horses against the venom of the tiger snake and then collecting that part of the horse’s blood which neutralizes this poison. The antivenom is purified and made into an injection for those people who may need it after being bitten by a tiger snake. Tiger snake antivenom is also the appropriate antivenom if you are bitten by a copperhead snake, a rough scaled snake or a member of the black snake family. -

Role of the Inflammasome in Defense Against Venoms
Role of the inflammasome in defense against venoms Noah W. Palm and Ruslan Medzhitov1 Department of Immunobiology, and Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06520 Contributed by Ruslan Medzhitov, December 11, 2012 (sent for review November 14, 2012) Venoms consist of a complex mixture of toxic components that are Large, multiprotein complexes responsible for the activation used by a variety of animal species for defense and predation. of caspase-1, termed inflammasomes, are activated in response Envenomation of mammalian species leads to an acute inflamma- to various infectious and noninfectious stimuli (14). The activa- tory response and can lead to the development of IgE-dependent tion of inflammasomes culminates in the autocatalytic cleavage venom allergy. However, the mechanisms by which the innate and activation of the proenzyme caspase-1 and the subsequent – immune system detects envenomation and initiates inflammatory caspase-1 dependent cleavage and noncanonical (endoplasmic- – fl and allergic responses to venoms remain largely unknown. Here reticulum and Golgi-independent) secretion of the proin am- matory cytokines IL-1β and IL-18, which lack leader sequences. we show that bee venom is detected by the NOD-like receptor fl family, pyrin domain-containing 3 inflammasome and can trigger In addition, activation of caspase-1 leads to a proin ammatory cell death termed pyroptosis. The NLRP3 inflammasome con- activation of caspase-1 and the subsequent processing and uncon- “ ” ventional secretion of the leaderless proinflammatory cytokine sists of the sensor protein NLRP3, the adaptor apoptosis-as- sociated speck-like protein (ASC) and caspase-1. Damage to IL-1β in macrophages. -

Pirra Jungku Project Species Guide
The Pirra Jungku Project is a collaboration between the Karajarri Rangers, Environs Kimberley Pirra Jungku Project and the Threatened Species Recovery Hub with funding from the Australian Government’s National Environmental Science Program and the species guide Western Australian Government’s NRM Program. Reptiles * Asterix means the animal can be tricky to ID. Take a good photo, or bring it back to camp for checking, but do this as a last resort. Don’t bring back any snakes, in case they are poisonous. Dragons Upright posture (stick their heads up), have small, rough scales, each leg has 5 clawed fingers/toes. MATT FROM MELBOURNE, AUSTRALIA CC BY 2.0 WIKIMEDIA COMMONS JESSSARAH MILLER LEGGE Slater’s ring-tailed dragon Central military dragon (Ctenophorus slaterii) (Ctenophorus isolepis) Rocky country. Reddish colour with black Sandy country. Very fast on ground. spots on back and dark rings on the tail. Reddish colour with white spots and stripes. JESSCHRISTOPHER MILLER WATSON CC BY SA 3.0 WIKIMEDIA COMMONS ARTHUR CHAPMAN NICOLAS RAKOTOPARE Pindan dragon Horner’s dragon Northern Pilbara tree dragon (Diporiphora pindan) (Lophognathus horneri) (Diporiphora vescus) Thin, slender body. Two long white stripes Ta-ta lizard. White stripe from lip to back legs. Lives in spinifex. Plain colour, sometimes down back that cross over black and orange Tiny white spot in ear. with orange tail, and long white and grey tiger stripes.* stripes down body.* CHRISTOPHERSARAH LEGGE WATSON CC BY SA 3.0 WIKIMEDIA COMMONS Dwarf bearded dragon (Pogona minor) Grey with flat body with spiny edges. Has small spines on either side of the jaw and on the back of the head.