Microbial Issues Encountered in Wastewater Treatment at Moorhead Factory and Remedial Measures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Infection Control
infection control JANICE CARR The above photo depicts an E.coli (ATCC 11775) biofilm grown on PC (polycarbonate) coupons using a CDC biofilm reactor. Microorganisms often colonize, and adhere strongly to living and non-living surfaces forming biofilms, and at times, demonstrate an increased resistance to antimicrobials. Biofilms on indwelling medical devices pose a serious threat to public health. BIOFILMS:BIOFILMS: Friend or Foe? By NICOLE KENNY, B.Sc, Assoc.Chem., Director of Professional & Technical Services, Virox Technologies Inc 38 Sanitation Canada - SEPTEMBER / OCTOBER 2006 JANICE CARR Scanning electron micrograph of a Staphylococcus biofilm on the inner surface of a needleless connector. A distinguishing characteristic of biofilms is the presence of extracellular polymeric substances, primarily polysaccharides, surrounding and encasing the cells. Here, there polysaccharides have been visualized by scanning electron microscopy. Picture yourself a con- “Why didn’t I listen to my mother and take Biofilms can be dangerous or benefi- testant on Jeopardy. Alex more science courses?” But in that split cial depending on where they are found Tribec has just asked you second you also remember a documen- and of which organisms they are com- to choose the category. tary you watched on CNN about whirl- prised. In industry, biofilms are responsi- You’re lagging behind the pool tubs and you know the answer. ble for billions of dollars in lost produc- leader by $400. All but “Alex, what are BIOFILMS?” tivity due to equipment damage, notori- one of the $500 questions ously famous for causing pipes to plug or Phave been taken, and the last category has THE ISSUE corrode. -

Functional Anatomy of Prokaryotes and Eukaryotes
FUNCTIONAL ANATOMY OF PROKARYOTES AND EUKARYOTES BY DR JAWAD NAZIR ASSISTANT PROFESSOR DEPARTMENT OF MICROBIOLOGY UNIVERSITY OF VETERINARY AND ANIMAL SCIENCES, LAHORE Prokaryotes vs Eukaryotes Prokaryote comes from the Greek words for pre-nucleus Eukaryote comes from the Greek words for true nucleus. Functional anatomy of prokaryotes Prokaryotes vs Eukaryotes Prokaryotes Eukaryotes One circular chromosome, not in Paired chromosomes, in nuclear a membrane membrane No histones Histones No organelles Organelles Peptidoglycan cell walls Polysaccharide cell walls Binary fission Mitotic spindle Functional anatomy of prokaryotes Size and shape Average size: 0.2 -1.0 µm 2 - 8 µm Basic shapes: Functional anatomy of prokaryotes Size and shape Pairs: diplococci, diplobacilli Clusters: staphylococci Chains: streptococci, streptobacilli Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Unusual shapes Star-shaped Stella Square Haloarcula Most bacteria are monomorphic A few are pleomorphic Genus: Stella Genus: Haloarcula Functional anatomy of prokaryotes Bacterial cell structure Structures external to cell wall Cell wall itself Structures internal to cell wall Functional anatomy of prokaryotes Glycocalyx Outside cell wall Usually sticky A capsule is neatly organized A slime layer is unorganized & loose Extracellular polysaccharide allows cell to attach Capsules prevent phagocytosis Association with diseases B. anthracis S. pneumoniae Functional anatomy of prokaryotes Flagella Outside cell wall Filament made of chains of flagellin Attached to a protein hook Anchored to the wall and membrane by the basal body Functional anatomy of prokaryotes Flagella Arrangement Functional anatomy of prokaryotes Bacterial motility Rotate flagella to run or tumble Move toward or away from stimuli (taxis) Flagella proteins are H antigens (e.g., E. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -
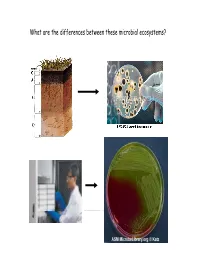
What Are the Differences Between These Microbial Ecosystems? Wwwwe Now Know That, Like Other Org Gm,Anisms, Bacteria Exhibit Social Behaviors
What are the differences between these microbial ecosystems? WWwwe now know that, like other org gm,anisms, bacteria exhibit social behaviors. Bacterial cell escaping for a rare moment of peace and quiet contemplation Sociomicrobiology I. Cell signaling A. Definition/description B. Intraspecific (within spp.): Myxococcus C. Interspecific (between spp.): Pseudomonas aureofaciens II. Biofilms A. Biofilm formation B. Planktonic cells vs. biofilm cells C. General characteristics, structures D. Biofilms as social entities Small diffusible molecules mediate bacterial communication O O N H AHL O O O N H O http://www.ted.com/index.php/talks/bonnie_bassler_on_how_bacteria_communicate.html Why cell-cell signaling in bacteria? Often, single cells in a population might benefit from knowing how many cells are present… “A multitude of bacteria are stronger than a few, thus by union are able overcome obstacles too great for few.” -- Dr. Erwin F. Smith, 1905 (Father of Plant Bacteriology) Pseudomonas aeruginosa in lungs Xylella fastidiosa in xylem Why cell-cell signaling in bacteria? Cell-cell signaling enables bacteria to coordinate behavior to respond quickly to environmental stimuli… such as: -presence of suitable host -change in nutrient availability -defense/competition against other microorganisms -many others! CmmiCommunica tion among btbacteri i:a: The example of Myxococcus xanthus, the Wolf Pack feeder of bacteria http://cmgm.stanford.edu/devbio/kaiserlab/about_myxo/about_myxococcus.html Cooperation among cells in a population: myxobacteria. The myxobacteria are Gram-negative, ubiquitous, soil-dwelling bacteria that are capable of multicellular, social behaviour. In the presence of nutrients, “swarms” of myxobacteria feed cooperatively by sharing extracellular digestive enzymes, and can prey on other bacteria. -

Introduction to Microbiology
Introduction to microbiology Prof. dr hab. Beata M. Sobieszczańska Department of Microbiology University of Medicine • http://www.lekarski.umed.wroc.pl/mikrobiologia • schedules, rules, important information • Consulting hours – teachers are always available for students during consulting hours or classes – apart from consulting hours – you must chase ! • Sick leaves (original) must be shown to the teacher just after an absence but not longer than after two weeks otherwise a sick note will not be honored - a copy of the sick note must be delivered to the secretary office • Class tests – 10 open questions • Terms: 1st, 2nd – if failed commission test from the whole material at the end of semester • Students with the average 4.8 will be released from the final exam • Presence on lectures and classes are obligatory • The final grade from classes is the average of all grades during semester Your best friend in this year: Medical Microbiology by Patrick R. Murray, Ken S. Rosenthal, Michael A. Pfaller Answer questions: • Name important cell wall structures of GP and GN bacteria • What is a role of these structures in human diseases? • Name other than bacterial cell wall structures and explain their role in bacterial pathogenicity • Do you understand the term pathogenicity? • Name five different genera GP and GN bacteria and indicate the colour they have after Gram staining Answer questions: • Name clinically important bacteria producing endospores – why endospores are important? • What is the difference between capsule and glycocalyx layer on GP bacteria? • What is axial filament? What role it plays? What bacteria produce axial filaments? • Name two types of pili and their role in bacterial pathogenicity Most bacteria come in one of three basic shapes: coccus, rod or bacillus & spiral MURRAY 7th ed. -
Gram Negative Bacterial Structure Explain the Medical Implications of Spore Formation
Lecture (3) Bacterial cell structure Objectives Enumerate Essential and non-essential bacterial cell components Describe the common anatomical structures found in bacteria and explain their function [flagella, pili, glycocalyx, capsule, endospores, cytoplasm, inclusions, chromosome, plasmids, cell membrane, and cell wall]. Compare gram positive and gram negative bacterial structure Explain the medical implications of spore formation. Bacterial cell structure •Cell wall Essential •Cytoplasmic Membrane •Cytoplasm Structures •Nuclear body •Capsule. Non Essential •Flagella •Pili structures •Inclusion granules Cell wall Cytoplasmic Essential structures membrane Any bacterial cell is composed of the following structures (Essential structures): 1. Cell wall. 2. Cytoplasmic membrane. 3. Cytoplasm. 4. Nuclear body. Nuceloid Cytoplasm Non Essential Structures Capsule Inclusion Some (Not all) bacteria may granules contain one or more of the following structures: 1. Capsule. 2. Flagella (single Flagellum) 3. Fimbria (pili). Pili 4. Inclusion granules Flagellum Cell wall Cytoplasmic THE CELL WALL membrane The cell wall is a rigid structure that surrounds the bacterial cell just outside of the plasma membrane. Nuceloid Cytoplasm Cell wall Structure Bacteria are classified according to their cell wall as: Gram positive or Gram negative. Peptidoglycan Polysaccharide chains The main structural component of the cell wall. Peptidoglycan is formed of carbohydrate + protein. It consists of long polysaccharide chains that are cross-linked by amino acid bridges. Gram positive Cell Wall In Gram-positive bacteria the peptidoglycan forms a thick layer external to the cell membrane. Cell wall of gram positive bacteria also contain Teichoic acid molecules. Gram negative Cell Wall In Gram-negative bacteria, the peptidoglycan layer is thin and is overlaid by an outer membrane. -

Staining the Purpose of Staining : We Stain Bacteria to Study There : A) Morphology and Arrangement
Staining The purpose of Staining : We stain bacteria to study there : A) Morphology and Arrangement . B)Differentiated bacteria to groups according to their biochemical composition of cell wall . C)Study structures of bacteria (capsule,flagella) . Stains are classified according to their functions into : 1)Simple stain (methylene blue , safranin) that help to stain the outlines of bacterial cells, giving one the characteristic shape ,size, and arrangement of the cells stained with the simple stain . 2)Differential stain (Gram stain ,acid fast stain) Differential stain will Differentiate between the two cells . 3)Special stains (capsule stain,flagella stain ) stained some structures of bacteria . Preparation of smear : 1. Clean the slide . 2. Place a loop full of water in the center of the slide . 3. Mix a small amount of bacteria using a loop with the water and spread it out . 4. Allow the slide to air dry. 5-Heat-fix the smear by passing the slide through the Benzen burner . 1)Simple stain : methylene blue stain 1. Prepare the smear . 2. Flood the slide with methylene blue stain for 3 mints . 3. Wash the slide with tap water gently,drain off excess water then let the slide dry in air or by using filter paper . 4. Exam it microscopically . 5. The bacteria will appear blue cells . 2) Differential stains : A)Gram staining . B)Ziehl Neelsen (Acid Fast ) staining : Is a differential stain used to identify acid-fast organisms as members of the genus Mycobacterium . Acid-fast organisms are characterized by wax-like,nearly impermeable cell walls;they contain mycolic acid and large amounts of fatty acids ,waxes,and complex lipids . -

Peptide Mediated Inhibition of Porphyromonas Gingivalis Dual and Three Species Biofilms
University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 12-2013 Peptide mediated inhibition of Porphyromonas gingivalis dual and three species biofilms. Naga Srinija Gummadi University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/etd Recommended Citation Gummadi, Naga Srinija, "Peptide mediated inhibition of Porphyromonas gingivalis dual and three species biofilms." (2013). Electronic Theses and Dissertations. Paper 545. https://doi.org/10.18297/etd/545 This Master's Thesis is brought to you for free and open access by ThinkIR: The University of Louisville's Institutional Repository. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of ThinkIR: The University of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. PEPTIDE MEDIATED INHIBITION OF PORPHYROMONAS GINGIVALIS DUAL AND THREE SPECIES BIOFILMS By: Naga Srinija Gummadi University of Louisville School of Dentistry A Thesis Submitted to the Faculty Of the School of Dentistry of the University of Louisville in Partial Fulfillment of the Requirements for the Degree of Master of Science Oral Biology School of Dentistry University of Louisville Louisville, KY December 2013 Copyright by Naga Srinija Gummadi in 2013 All Rights Reserved PEPTIDE MEDIATED INHIBITION OF PORPHYROMONAS GINGIVALIS DUAL AND THREE SPECIES BIOFILMS By: Naga Srinija Gummadi, B.D.S., Dr. N.T.R University of Health Sciences, 2009 Thesis Approved on November 27, 2013 By the following Thesis Committee ______________________________ Dr. Donald R. Demuth, Ph.D. -

Review of Microbially Influenced Corrosion of High-Level Waste
CNWRA 93-014 A S S. l ' -S I& 0X- 0,,,, al-s~~~~~~~~~~~ _ Prepared for Nuclear Regulatory Commission Contract NRC-02-88-005 Prepared by Center for Nuclear Waste Regulatory Analyses San Antonio, Texas June 1993 CNWRA 93-014 A REVIEW OF THE POTENTIAL FOR MICROBIALLY INFLUENCED CORROSION OF HIGH-LEVEL NUCLEAR WASTE CONTAINERS Prepared for Nuclear Regulatory Commission Contract NRC-02-88-005 Prepared by Gill Geesey Department of Microbiology Montana State University Bozeman, Montana Edited by Gustavo A. Cragnolino Center for Nuclear Waste Regulatory Analyses San Antonio, Texas June 1993 RECEIVED JUN 281993 CNWRA-WO PREVIOUS REPORTS IN SERIES Number Name Date Issued CNWRA 91-004 A Review of Localized Corrosion of High-Level Nuclear Waste Container Materials - I April 1991 CNWRA 91-008 Hydrogen Embrittlement of Candidate Container Materials June 1991 CNWRA 92-021 A Review of Stress Corrosion Cracking of High-Level Nuclear Waste Container Materials - I August 1992 CNWRA 93-003 Long-Term Stability of High-Level Nuclear Waste Container Materials: I - Thermal Stability of Alloy 825 February 1993 CNWRA 93-004 Experimental Investigations of Localized Corrosion of High-Level Waste Container Materials February 1993 ii ABSTRACT The potential for microbially influenced corrosion (MIC) of the candidate and alternate container materials for the proposed Yucca Mountain repository site is examined on the basis of an extensive review of the literature. A brief description of the environmental conditions expected outside the waste packages, in terms of the geology, hydrology, water chemistry, radiation, temperature, and moisture content, is followed by a detailed discussion regarding the characteristics of microbial life in subsurface environments. -

Prokaryotic Cell Features
Prokaryotic Cell Features Size The small size of prokaryotic cells affects their physiology, growth rate, and ecology. Due to their small cell size, most prokaryotes have the highest surface area–to–volume ratio of any cells. This characteristic aids in nutrient and waste exchange with the environment. As a cell increases in size, its surface area-to-volume ratio decreases For a sphere, S / V = 3/r Prokaryotes maintain a high surface area to volume ratio by their smallness. 1. TRANSPORT RATE: Efficient transport of raw materials in and wastes out 2. GROWTH RATE: Nutrient exchange limits growth rates. Many prokaryotes = high metabolic activity (fast growth, reproductive rate). 3. EVOLUTIONARY RATE: Given the same amount of nutrients, small cells can have more individuals in a population than larger cells. Thus, more cell division, and more mutation, more evolutionary change. Eukaryotes larger cell size (in general) Different answers for the transport problem 1. lots of internal membrane channels, invaginations and surface area 2. cytoplasmic streaming 3. organelles (compartmentalize cell functions) 4. bulk uptake - endocytosis (phagocytosis/pinocytosis) Introducing a prokaryote that is one million times bigger than E. coli! Sturgeonfish symbiont. Epulopiscium fiseloni 600 µm (0.6 mm) long 20 - 40 µm long Individual flagella twist together to form “thick” flagella Largest known bacterium: Thiomargarita namibiensis Some nearly 1 mm wide Large central vacuole (dark) contains nitrate sequestered to use in the oxidation of reduced sulfur compounds. String of pearls: reflective sulfur granules How do we see microbes? 1. Light Microscopy Up to 1,500 X magnification (yours in lab: 1000 X) Limit of resolving power is ~0.2 µM, or about 1/3 the width of an average bacterial cell Light gathering ability decreases as magnification increases Immersion oil helps gather light rays that would otherwise be lost from specimen How do we see microbes? 2. -

Algal and Cyanobacterial Saline Biofilms of the Grande Coastal Lagoon, Lima, Peru
Natural Resources and Environmental Issues Volume 15 Saline Lakes Around the World: Unique Systems with Unique Values Article 23 2009 Algal and cyanobacterial saline biofilms of the Grande Coastal Lagoon, Lima, Peru Haydee Montoya Natural History Museum, UNMSM, and Biological Sciences, Ricardo Palma University, Lima, Peru Follow this and additional works at: https://digitalcommons.usu.edu/nrei Recommended Citation Montoya, Haydee (2009) "Algal and cyanobacterial saline biofilms of the Grande Coastal Lagoon, Lima, Peru," Natural Resources and Environmental Issues: Vol. 15 , Article 23. Available at: https://digitalcommons.usu.edu/nrei/vol15/iss1/23 This Article is brought to you for free and open access by the Journals at DigitalCommons@USU. It has been accepted for inclusion in Natural Resources and Environmental Issues by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Montoya: Algal and cyanobacterial biofilms of the Grande Coastal Lagoon Algal and Cyanobacterial Saline Biofilms of the Grande Coastal Lagoon, Lima, Peru Haydee Montoya1 1Natural History Museum, UNMSM, and Biological Sciences Faculty, Ricardo Palma University, Av. Arenales 1256, Apartado 14-0434, Lima 14, Perú, E-mail: [email protected] ABSTRACT coexist at an interface, with a distinct macromolecular matrix typically attached to a surface in which complex Tropical coastal wetland ecosystems are widely distributed food webs occur (Davey & O’Toole 2000; Larson & Passy in arid regions. The Grande coastal lagoon in Peru’s central 2005; De Vicente et al. 2006). Photosynthetic activity by plain is shallow, eutrophic and alkaline, exposed to the benthic microalgae is the primary source of fixed carbon in annual hydrological regime with flooding and desiccation shallow aquatic ecosystems. -

Chapter 4 – Prokaryotic Profiles External Structures Flagella
Chapter 4 – Prokaryotic • Prokaryotes are unicellular organisms Profiles • Prokaryotes include two small groups of Topics: organisms - the archaeobacteria and the –External Structures photosynthetic cyanobacteria plus the large group of true bacteria or eubacteria –Cell Envelope –Internal Structures •Prokaryotes are generally small - in the –Cell Shapes, Arrangement, and range of 0.2 to 2.0 µµµm. However, there are Sizes exceptions. Cyanobacteria may be 60 µµµm long External Structures Flagella • Flagella • Composed of protein subunits • Pili and fimbriae • Role - Motility (chemotaxis) • Glycocalyx • Varied arrangement (ex. Monotrichous, lophotrichous, amphitrichous) • Responsible for swarming in P. aeruginosa 1 Three main parts of the flagella include the basal body, hook, and filament. 3 2 1 There are 4 types of flagellar arrangement in bacteria – important for movement and useful in Mono Lopho diagnosing particular infections Monotrichous Amphitrichous Lophotrichous Peritrichous Amphi Peri The rotation of the flagella enables bacteria Associated with flagella is the to be motile phenomenon of chemotaxis – random ‘tumbles’ followed by directional ‘runs’ Chemotaxis - cells have the capability of responding to chemical attractants 2 Chemotaxis - response to chemical signals Periplasmic flagellum or Axial filament –present in some spirochetes RUN TUMBLE Pili and fimbriae - Pili are formed on certain bacterial cells and are important for • Attachment bacteriophage attachment, conjugation bridges for gene transfer • Mating (Conjugation)