Review of Microbially Influenced Corrosion of High-Level Waste
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sulfuric Acid Corrosion to Simulate Microbial Influenced Corrosion on Stainless
Sulfuric Acid Corrosion to Simulate Microbial Influenced Corrosion on Stainless Steel 316L by Jacob T. Miller Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Engineering in the Chemical Engineering Program YOUNGSTOWN STATE UNIVERSITY December, 2017 Sulfuric Acid Corrosion to Simulate Microbial Influenced Corrosion on Stainless Steel 316L Jacob T. Miller I hereby release thesis to the public. I understand that this thesis will be made available from the OhioLINK ETD Center and the Maag Library Circulation Desk for public access. I also authorize the University or other individuals to make copies of this thesis as needed for scholarly research. Signature: Jacob T. Miller, Student Date Approvals: Dr. Holly J. Martin, Thesis Advisor Date Dr. Pedro Cortes, Committee Member Date Dr. Brett P. Conner, Committee Member Date Dr. Salvatore A. Sanders, Dean of Graduate Studies Date Abstract The continued improvement of additive manufacturing (3D printing) is progressively eliminating the geometric limitations of traditional subtractive processes. Because parts are built up in thin layers, such as in processes like Laser Powder Bed Fusion, complex parts can be manufactured easily. However, this manufacturing method likely causes the parts to have rougher surfaces and decreased density compared to their traditional counterparts. The effect of this difference has not been researched thoroughly, but may have a significant impact on the properties of the parts. For example, the 3D printed parts could more easily collect micro-organisms that produce sulfuric acid as byproducts of their metabolic processes. Uninhibited microbial growth on the sample surface could produce enough sulfuric acid to degrade the parts through hydrogen embrittlement. -

Sulphate-Reducing Bacteria's Response to Extreme Ph Environments and the Effect of Their Activities on Microbial Corrosion
applied sciences Review Sulphate-Reducing Bacteria’s Response to Extreme pH Environments and the Effect of Their Activities on Microbial Corrosion Thi Thuy Tien Tran 1 , Krishnan Kannoorpatti 1,* , Anna Padovan 2 and Suresh Thennadil 1 1 Energy and Resources Institute, College of Engineering, Information Technology and Environment, Charles Darwin University, Darwin, NT 0909, Australia; [email protected] (T.T.T.T.); [email protected] (S.T.) 2 Research Institute for the Environment and Livelihoods, College of Engineering, Information Technology and Environment, Charles Darwin University, Darwin, NT 0909, Australia; [email protected] * Correspondence: [email protected] Abstract: Sulphate-reducing bacteria (SRB) are dominant species causing corrosion of various types of materials. However, they also play a beneficial role in bioremediation due to their tolerance of extreme pH conditions. The application of sulphate-reducing bacteria (SRB) in bioremediation and control methods for microbiologically influenced corrosion (MIC) in extreme pH environments requires an understanding of the microbial activities in these conditions. Recent studies have found that in order to survive and grow in high alkaline/acidic condition, SRB have developed several strategies to combat the environmental challenges. The strategies mainly include maintaining pH homeostasis in the cytoplasm and adjusting metabolic activities leading to changes in environmental pH. The change in pH of the environment and microbial activities in such conditions can have a Citation: Tran, T.T.T.; Kannoorpatti, significant impact on the microbial corrosion of materials. These bacteria strategies to combat extreme K.; Padovan, A.; Thennadil, S. pH environments and their effect on microbial corrosion are presented and discussed. -

Infection Control
infection control JANICE CARR The above photo depicts an E.coli (ATCC 11775) biofilm grown on PC (polycarbonate) coupons using a CDC biofilm reactor. Microorganisms often colonize, and adhere strongly to living and non-living surfaces forming biofilms, and at times, demonstrate an increased resistance to antimicrobials. Biofilms on indwelling medical devices pose a serious threat to public health. BIOFILMS:BIOFILMS: Friend or Foe? By NICOLE KENNY, B.Sc, Assoc.Chem., Director of Professional & Technical Services, Virox Technologies Inc 38 Sanitation Canada - SEPTEMBER / OCTOBER 2006 JANICE CARR Scanning electron micrograph of a Staphylococcus biofilm on the inner surface of a needleless connector. A distinguishing characteristic of biofilms is the presence of extracellular polymeric substances, primarily polysaccharides, surrounding and encasing the cells. Here, there polysaccharides have been visualized by scanning electron microscopy. Picture yourself a con- “Why didn’t I listen to my mother and take Biofilms can be dangerous or benefi- testant on Jeopardy. Alex more science courses?” But in that split cial depending on where they are found Tribec has just asked you second you also remember a documen- and of which organisms they are com- to choose the category. tary you watched on CNN about whirl- prised. In industry, biofilms are responsi- You’re lagging behind the pool tubs and you know the answer. ble for billions of dollars in lost produc- leader by $400. All but “Alex, what are BIOFILMS?” tivity due to equipment damage, notori- one of the $500 questions ously famous for causing pipes to plug or Phave been taken, and the last category has THE ISSUE corrode. -

Beating the Bugs: Roles of Microbial Biofilms in Corrosion
Beating the bugs: roles of microbial biofilms in corrosion The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Li, Kwan, Matthew Whitfield, and Krystyn J. Van Vliet. "Beating the bugs: roles of microbial biofilms in corrosion." Corrosion Reviews 321, 3-6 (2013); © 2013, by Walter de Gruyter Berlin Boston. All rights reserved. As Published https://dx.doi.org/10.1515/CORRREV-2013-0019 Publisher Walter de Gruyter GmbH Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/125679 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike Detailed Terms http://creativecommons.org/licenses/by-nc-sa/4.0/ Beating the bugs: Roles of microbial biofilms in corrosion Kwan Li∗,‡, Matthew Whitfield∗,‡, and Krystyn J. Van Vliet∗,† ∗Department of Materials Science and Engineering and †Department of Biological Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 USA ‡These author contributed equally to this work Abstract Microbiologically influenced corrosion is a complex type of environmentally assisted corrosion. Though poorly understood and challenging to ameliorate, it is increasingly appreciated that MIC accelerates failure of metal alloys, including steel pipeline. His- torically, this type of material degradation process has been treated from either an electrochemical materials perspective or a microbiological perspective. Here, we re- view the current understanding of MIC mechanisms for steel – particularly those in sour environments relevant to fossil fuel recovery and processing – and outline the role of the bacterial biofilm in both corrosion processes and mitigation responses. Keywords: biofilm; sulfate-reducing bacteria (SRB); microbiologically influenced cor- rosion (MIC) 1 Introduction Microbiologically influenced corrosion (MIC) can accelerate mechanical failure of metals in a wide range of environments ranging from oil and water pipelines and machinery to biomedical devices. -

Functional Anatomy of Prokaryotes and Eukaryotes
FUNCTIONAL ANATOMY OF PROKARYOTES AND EUKARYOTES BY DR JAWAD NAZIR ASSISTANT PROFESSOR DEPARTMENT OF MICROBIOLOGY UNIVERSITY OF VETERINARY AND ANIMAL SCIENCES, LAHORE Prokaryotes vs Eukaryotes Prokaryote comes from the Greek words for pre-nucleus Eukaryote comes from the Greek words for true nucleus. Functional anatomy of prokaryotes Prokaryotes vs Eukaryotes Prokaryotes Eukaryotes One circular chromosome, not in Paired chromosomes, in nuclear a membrane membrane No histones Histones No organelles Organelles Peptidoglycan cell walls Polysaccharide cell walls Binary fission Mitotic spindle Functional anatomy of prokaryotes Size and shape Average size: 0.2 -1.0 µm 2 - 8 µm Basic shapes: Functional anatomy of prokaryotes Size and shape Pairs: diplococci, diplobacilli Clusters: staphylococci Chains: streptococci, streptobacilli Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Unusual shapes Star-shaped Stella Square Haloarcula Most bacteria are monomorphic A few are pleomorphic Genus: Stella Genus: Haloarcula Functional anatomy of prokaryotes Bacterial cell structure Structures external to cell wall Cell wall itself Structures internal to cell wall Functional anatomy of prokaryotes Glycocalyx Outside cell wall Usually sticky A capsule is neatly organized A slime layer is unorganized & loose Extracellular polysaccharide allows cell to attach Capsules prevent phagocytosis Association with diseases B. anthracis S. pneumoniae Functional anatomy of prokaryotes Flagella Outside cell wall Filament made of chains of flagellin Attached to a protein hook Anchored to the wall and membrane by the basal body Functional anatomy of prokaryotes Flagella Arrangement Functional anatomy of prokaryotes Bacterial motility Rotate flagella to run or tumble Move toward or away from stimuli (taxis) Flagella proteins are H antigens (e.g., E. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -
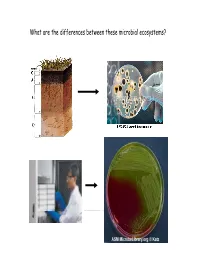
What Are the Differences Between These Microbial Ecosystems? Wwwwe Now Know That, Like Other Org Gm,Anisms, Bacteria Exhibit Social Behaviors
What are the differences between these microbial ecosystems? WWwwe now know that, like other org gm,anisms, bacteria exhibit social behaviors. Bacterial cell escaping for a rare moment of peace and quiet contemplation Sociomicrobiology I. Cell signaling A. Definition/description B. Intraspecific (within spp.): Myxococcus C. Interspecific (between spp.): Pseudomonas aureofaciens II. Biofilms A. Biofilm formation B. Planktonic cells vs. biofilm cells C. General characteristics, structures D. Biofilms as social entities Small diffusible molecules mediate bacterial communication O O N H AHL O O O N H O http://www.ted.com/index.php/talks/bonnie_bassler_on_how_bacteria_communicate.html Why cell-cell signaling in bacteria? Often, single cells in a population might benefit from knowing how many cells are present… “A multitude of bacteria are stronger than a few, thus by union are able overcome obstacles too great for few.” -- Dr. Erwin F. Smith, 1905 (Father of Plant Bacteriology) Pseudomonas aeruginosa in lungs Xylella fastidiosa in xylem Why cell-cell signaling in bacteria? Cell-cell signaling enables bacteria to coordinate behavior to respond quickly to environmental stimuli… such as: -presence of suitable host -change in nutrient availability -defense/competition against other microorganisms -many others! CmmiCommunica tion among btbacteri i:a: The example of Myxococcus xanthus, the Wolf Pack feeder of bacteria http://cmgm.stanford.edu/devbio/kaiserlab/about_myxo/about_myxococcus.html Cooperation among cells in a population: myxobacteria. The myxobacteria are Gram-negative, ubiquitous, soil-dwelling bacteria that are capable of multicellular, social behaviour. In the presence of nutrients, “swarms” of myxobacteria feed cooperatively by sharing extracellular digestive enzymes, and can prey on other bacteria. -

Laboratory Based Investigation of Stress Corrosion Cracking of Cable Bolts
Laboratory Based Investigation of Stress Corrosion Cracking of Cable Bolts Saisai Wu A thesis in fulfilment of the requirements for the degree of Doctor of Philosophy School of Mining Engineering Faculty of Engineering July 2018 THE UNIVERSITY OF NEW SOUTH WALES Thesis/Dissertation Sheet Surname or Family name: Wu Other name/s: First name: Saisai Abbreviation for degree as given in the University calendar: PhD School: Mining Engineering Faculty: Engineering Title: Laboratory-Based Investigation of Stress Corrosion Cracking Of Cable Bolts Abstract 350 words maximum Premature failure of cable bolts due to stress corrosion cracking (SCC) in underground excavations is a worldwide problem with limited cost-effective solutions at present. To determine the cause and mechanism of SCC, identify potential technologies and eventually avoid catastrophic failure of cable bolts, a two-step methodology was implemented: (i) a long-term test using groundwater collected from underground mines, and (ii) an accelerated test using an acidified solution. Laboratory experimentation on both representative coupon and full-size cable bolt specimens was conducted. In the long-term tests, simulated underground environments were recreated in ‘corrosion cells’ which contained a newly designed cable bolt coupon together with a mixture of groundwater, coal and clay, to measure the potential for developing SCC. The incidence of SCC failures was not related to groundwater alone. Geomaterials in the corrosion cells accelerated the corrosion of cable bolts by increasing the concentrations of total dissolved solids and electrical conductivity of the water. Following this, an acidic solution containing sulphide, synthesised based on the chemical properties of groundwater from twelve Australian underground mines, was used as the testing solution for the accelerated tests. -

Introduction to Microbiology
Introduction to microbiology Prof. dr hab. Beata M. Sobieszczańska Department of Microbiology University of Medicine • http://www.lekarski.umed.wroc.pl/mikrobiologia • schedules, rules, important information • Consulting hours – teachers are always available for students during consulting hours or classes – apart from consulting hours – you must chase ! • Sick leaves (original) must be shown to the teacher just after an absence but not longer than after two weeks otherwise a sick note will not be honored - a copy of the sick note must be delivered to the secretary office • Class tests – 10 open questions • Terms: 1st, 2nd – if failed commission test from the whole material at the end of semester • Students with the average 4.8 will be released from the final exam • Presence on lectures and classes are obligatory • The final grade from classes is the average of all grades during semester Your best friend in this year: Medical Microbiology by Patrick R. Murray, Ken S. Rosenthal, Michael A. Pfaller Answer questions: • Name important cell wall structures of GP and GN bacteria • What is a role of these structures in human diseases? • Name other than bacterial cell wall structures and explain their role in bacterial pathogenicity • Do you understand the term pathogenicity? • Name five different genera GP and GN bacteria and indicate the colour they have after Gram staining Answer questions: • Name clinically important bacteria producing endospores – why endospores are important? • What is the difference between capsule and glycocalyx layer on GP bacteria? • What is axial filament? What role it plays? What bacteria produce axial filaments? • Name two types of pili and their role in bacterial pathogenicity Most bacteria come in one of three basic shapes: coccus, rod or bacillus & spiral MURRAY 7th ed. -

Characterization of Sulfur Oxidizing Bacteria Related to Biogenic Sulfuric Acid Corrosion in Sludge Digesters Bettina Huber, Bastian Herzog, Jörg E
Huber et al. BMC Microbiology (2016) 16:153 DOI 10.1186/s12866-016-0767-7 RESEARCH ARTICLE Open Access Characterization of sulfur oxidizing bacteria related to biogenic sulfuric acid corrosion in sludge digesters Bettina Huber, Bastian Herzog, Jörg E. Drewes*, Konrad Koch and Elisabeth Müller Abstract Background: Biogenic sulfuric acid (BSA) corrosion damages sewerage and wastewater treatment facilities but is not well investigated in sludge digesters. Sulfur/sulfide oxidizing bacteria (SOB) oxidize sulfur compounds to sulfuric acid, inducing BSA corrosion. To obtain more information on BSA corrosion in sludge digesters, microbial communities from six different, BSA-damaged, digesters were analyzed using culture dependent methods and subsequent polymerase chain reaction denaturing gradient gel electrophoresis (PCR-DGGE). BSA production was determined in laboratory scale systems with mixed and pure cultures, and in-situ with concrete specimens from the digester headspace and sludge zones. Results: The SOB Acidithiobacillus thiooxidans, Thiomonas intermedia,andThiomonas perometabolis were cultivated and compared to PCR-DGGE results, revealing the presence of additional acidophilic and neutrophilic SOB. Sulfate concentrations of 10–87 mmol/L after 6–21 days of incubation (final pH 1.0–2.0)inmixedcultures,andupto 433 mmol/L after 42 days (final pH <1.0) in pure A. thiooxidans cultures showed huge sulfuric acid production potentials. Additionally, elevated sulfate concentrations in the corroded concrete of the digester headspace in contrast to the concrete of the sludge zone indicated biological sulfur/sulfide oxidation. Conclusions: The presence of SOB and confirmation of their sulfuric acid production under laboratory conditions reveal that these organisms might contribute to BSA corrosion within sludge digesters. -
Gram Negative Bacterial Structure Explain the Medical Implications of Spore Formation
Lecture (3) Bacterial cell structure Objectives Enumerate Essential and non-essential bacterial cell components Describe the common anatomical structures found in bacteria and explain their function [flagella, pili, glycocalyx, capsule, endospores, cytoplasm, inclusions, chromosome, plasmids, cell membrane, and cell wall]. Compare gram positive and gram negative bacterial structure Explain the medical implications of spore formation. Bacterial cell structure •Cell wall Essential •Cytoplasmic Membrane •Cytoplasm Structures •Nuclear body •Capsule. Non Essential •Flagella •Pili structures •Inclusion granules Cell wall Cytoplasmic Essential structures membrane Any bacterial cell is composed of the following structures (Essential structures): 1. Cell wall. 2. Cytoplasmic membrane. 3. Cytoplasm. 4. Nuclear body. Nuceloid Cytoplasm Non Essential Structures Capsule Inclusion Some (Not all) bacteria may granules contain one or more of the following structures: 1. Capsule. 2. Flagella (single Flagellum) 3. Fimbria (pili). Pili 4. Inclusion granules Flagellum Cell wall Cytoplasmic THE CELL WALL membrane The cell wall is a rigid structure that surrounds the bacterial cell just outside of the plasma membrane. Nuceloid Cytoplasm Cell wall Structure Bacteria are classified according to their cell wall as: Gram positive or Gram negative. Peptidoglycan Polysaccharide chains The main structural component of the cell wall. Peptidoglycan is formed of carbohydrate + protein. It consists of long polysaccharide chains that are cross-linked by amino acid bridges. Gram positive Cell Wall In Gram-positive bacteria the peptidoglycan forms a thick layer external to the cell membrane. Cell wall of gram positive bacteria also contain Teichoic acid molecules. Gram negative Cell Wall In Gram-negative bacteria, the peptidoglycan layer is thin and is overlaid by an outer membrane. -

Microbial Issues Encountered in Wastewater Treatment at Moorhead Factory and Remedial Measures
MICROBIAL ISSUES ENCOUNTERED IN WASTEWATER TREATMENT AT MOORHEAD FACTORY AND REMEDIAL MEASURES Indrani S. Samaraweera*1, Terry D. McGillivray1, Diane L. Rheault1 and Dennis Burthwick2 American Crystal Sugar Company, Technical Services Center 11700 N. 11th Street and 22500 N. 11th Street, Moorhead, MN 56560 Introduction: Wastewater treatment is an integral part of processing of sugar beets in the sugar industry. American Crystal Sugar Company (ACS) has five factories. Three of these factories, Moorhead (MHD), Hillsboro (HLB), and East Grand Forks (EGF) each have a 6.7 million gallon anaerobic contactor, aerobic basin, and ponds for processing of wastewater while the other two factories have lagoons and wetlands for the treatment of their wastewater. During the 2007/2008 campaign our efforts were focused on microbial issues in wastewater treatment at the MHD factory. Therefore, this paper deals with problems encountered with filamentous bacteria, poor settling in treatment of the high strength wastewater and studies to circumvent these problems. In addition some differences observed in the MHD and HLB anaerobic systems will also be discussed. Materials and Methods: A) Microbiology 1) Sample collection Weekly samples of wastewater were obtained aseptically in sterile screw cap containers from each of three locations: a) anaerobic influent from the new covered wastewater pond, b) anaerobic tank or anaerobic contactor, and c) aerobic basin or activated sludge system. These samples were observed microscopically at the ACS Technical Services Center Microbiology Lab. Samples from similar locations in the wastewater treatment systems at the Hillsboro and East Grand Forks factories were intermittently obtained for comparative purposes. 2) Microscopy and Photography i) Wet Mounts, Staining, Floc formation and Higher Life Forms – Separate wastewater (WW) wet mounts on slides were observed microscopically with or without a drop of lactophenol cotton blue and/or India ink stain.