Prokaryotic Cell Features
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prokaryotes (Domains Bacteria & Archaea)
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

The First Cells Were Most Likely Very Simple Prokaryotic Forms. Ra- Spirochetes
T HE O RIGIN OF E UKARYOTIC C ELLS The first cells were most likely very simple prokaryotic forms. Ra- spirochetes. Ingestion of prokaryotes that resembled present-day diometric dating indicates that the earth is 4 to 5 billion years old cyanobacteria could have led to the endosymbiotic development of and that prokaryotes may have arisen more than 3.5 billion years chloroplasts in plants. ago. Eukaryotes are thought to have first appeared about 1.5 billion Another hypothesis for the evolution of eukaryotic cells proposes years ago. that the prokaryotic cell membrane invaginated (folded inward) to en- The eukaryotic cell might have evolved when a large anaerobic close copies of its genetic material (figure 1b). This invagination re- (living without oxygen) amoeboid prokaryote ingested small aerobic (liv- sulted in the formation of several double-membrane-bound entities ing with oxygen) bacteria and stabilized them instead of digesting them. (organelles) in a single cell. These entities could then have evolved This idea is known as the endosymbiont hypothesis (figure 1a) and into the eukaryotic mitochondrion, nucleus, and chloroplasts. was first proposed by Lynn Margulis, a biologist at Boston Univer- Although the exact mechanism for the evolution of the eu- sity. (Symbiosis is an intimate association between two organisms karyotic cell will never be known with certainty, the emergence of of different species.) According to this hypothesis, the aerobic bac- the eukaryotic cell led to a dramatic increase in the complexity and teria developed into mitochondria, which are the sites of aerobic diversity of life-forms on the earth. At first, these newly formed eu- respiration and most energy conversion in eukaryotic cells. -

Prokaryotes, Protists, Reconstructing the Photosynthesis, Evolution of Living Things
Prokaryotes, Protists, Reconstructing the Photosynthesis, evolution of living things Endosymbiosis • Systematists study evolutionary relationships Ch 28 & 29 •Look for shared derived (=different from ancestor) traits to group organisms • Evidence used: morphology, 26 February 2009 development, and molecular data (especially DNA sequences) ECOL 182R UofA K. E. Bonine 1 2 Why can’t we figure it out perfectly? • More distant history is obscured by more changes trypanosomes • Among oldest lineages of Bacteria and Archaea in particular, lots of “lateral gene transfer.” Makes it difficult to infer relationships from phylogeny of red blood cells single genes. 3 4 Early prokaryote fossil Diversity of Prokaryotes Bacteria & Archaea 5 6 1 What are microbes? Life can be divided into 3 domains • Only a minority make us sick •Robert Koch, Germ Theory of Disease • In ordinary English, might be anything small 3.8bya –bacteria 1.5bya –yeast –protists – viruses •Prokaryotes = bacteria + archaea • In science, classify by evolutionary • Prokaryote was ancestral and only form for relationships… 7 billions of years 8 Eukarya Scheme has been revised before: Haeckel (1894) Whittaker (1959) Woese (1977) Woese (1990) Three kingdoms Five kingdoms Six kingdoms Three domains Eubacteria Bacteria Monera (prokaryotes) Protista Archaebacteria Archaea Protista Protista Fungi Fungi Plantae Eukarya are Prokaryotes monophyletic, Plantae Plantae Animalia Animalia Animalia paraphyleticparaphyletic, polyphyletic? 9 modified from Wikipedia 10 Shared by all 3 domains Unique to -

Infection Control
infection control JANICE CARR The above photo depicts an E.coli (ATCC 11775) biofilm grown on PC (polycarbonate) coupons using a CDC biofilm reactor. Microorganisms often colonize, and adhere strongly to living and non-living surfaces forming biofilms, and at times, demonstrate an increased resistance to antimicrobials. Biofilms on indwelling medical devices pose a serious threat to public health. BIOFILMS:BIOFILMS: Friend or Foe? By NICOLE KENNY, B.Sc, Assoc.Chem., Director of Professional & Technical Services, Virox Technologies Inc 38 Sanitation Canada - SEPTEMBER / OCTOBER 2006 JANICE CARR Scanning electron micrograph of a Staphylococcus biofilm on the inner surface of a needleless connector. A distinguishing characteristic of biofilms is the presence of extracellular polymeric substances, primarily polysaccharides, surrounding and encasing the cells. Here, there polysaccharides have been visualized by scanning electron microscopy. Picture yourself a con- “Why didn’t I listen to my mother and take Biofilms can be dangerous or benefi- testant on Jeopardy. Alex more science courses?” But in that split cial depending on where they are found Tribec has just asked you second you also remember a documen- and of which organisms they are com- to choose the category. tary you watched on CNN about whirl- prised. In industry, biofilms are responsi- You’re lagging behind the pool tubs and you know the answer. ble for billions of dollars in lost produc- leader by $400. All but “Alex, what are BIOFILMS?” tivity due to equipment damage, notori- one of the $500 questions ously famous for causing pipes to plug or Phave been taken, and the last category has THE ISSUE corrode. -

Prokaryote Vs. Eukaryote Campaign Biology Name: ______Period: ______
Prokaryote vs. Eukaryote Campaign Biology Name: ___________________________________________ Period: ____________ Prokaryotes vs. Eukaryotes: A Campaign for Election as Coolest Cell Type! Purpose: While prokaryotes and eukaryotes share some similar structures and characteristics of life, the two lead some pretty different lives! Your job in this assignment is to create a campaign poster and a campaign platform commercial for a prokaryote or a eukaryote as they campaign to be elected coolest cell type! Directions: Each group will be assigned a campaign platform from those listed below. Use the resources listed (as well as any others you might find) to understand why your platform makes your cell type the coolest. Create a campaign poster (using one slide on a Google Presentation, shared between partners, and submitted by due date via a form) and a short campaign platform commercial (given as a speech in class) using the attached rubric. Prokaryotic campaign platforms: 1. Ability to form endospores http://goo.gl/Y1tUK http://goo.gl/DhVNe 2. Ability to form biofilms with other cells http://goo.gl/umy7Z http://goo.gl/m9d5W 3. Quorum sensing neighboring cells http://goo.gl/DSiJU http://goo.gl/IFFtA 4. Production of bacteriocins http://goo.gl/lW75y http://goo.gl/WzFv1 5. Endosymbiotic theory http://goo.gl/6VIba http://goo.gl/uSYh4 Eukaryotic campaign platforms: 6. Contain organelles that have their own genetic material in addition to that found in the nucleus http://goo.gl/6VIba http://goo.gl/Nqesq 7. Vesicles http://goo.gl/bwF3x http://goo.gl/j3qY3 8. Membrane bound organelles that facilitate transport (endomembrane system) http://goo.gl/j3qY3 http://goo.gl/i4brZ 9. -

Functional Anatomy of Prokaryotes and Eukaryotes
FUNCTIONAL ANATOMY OF PROKARYOTES AND EUKARYOTES BY DR JAWAD NAZIR ASSISTANT PROFESSOR DEPARTMENT OF MICROBIOLOGY UNIVERSITY OF VETERINARY AND ANIMAL SCIENCES, LAHORE Prokaryotes vs Eukaryotes Prokaryote comes from the Greek words for pre-nucleus Eukaryote comes from the Greek words for true nucleus. Functional anatomy of prokaryotes Prokaryotes vs Eukaryotes Prokaryotes Eukaryotes One circular chromosome, not in Paired chromosomes, in nuclear a membrane membrane No histones Histones No organelles Organelles Peptidoglycan cell walls Polysaccharide cell walls Binary fission Mitotic spindle Functional anatomy of prokaryotes Size and shape Average size: 0.2 -1.0 µm 2 - 8 µm Basic shapes: Functional anatomy of prokaryotes Size and shape Pairs: diplococci, diplobacilli Clusters: staphylococci Chains: streptococci, streptobacilli Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Unusual shapes Star-shaped Stella Square Haloarcula Most bacteria are monomorphic A few are pleomorphic Genus: Stella Genus: Haloarcula Functional anatomy of prokaryotes Bacterial cell structure Structures external to cell wall Cell wall itself Structures internal to cell wall Functional anatomy of prokaryotes Glycocalyx Outside cell wall Usually sticky A capsule is neatly organized A slime layer is unorganized & loose Extracellular polysaccharide allows cell to attach Capsules prevent phagocytosis Association with diseases B. anthracis S. pneumoniae Functional anatomy of prokaryotes Flagella Outside cell wall Filament made of chains of flagellin Attached to a protein hook Anchored to the wall and membrane by the basal body Functional anatomy of prokaryotes Flagella Arrangement Functional anatomy of prokaryotes Bacterial motility Rotate flagella to run or tumble Move toward or away from stimuli (taxis) Flagella proteins are H antigens (e.g., E. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -

Cell Theory VOCABULARY Unit 2: Cells Unit 2: > RE B
3.1 Cell Theory KEY CONCEPT Cells are the basic unit of life. VOCABULARY cell theory MAIN IDEAS cytoplasm Early studies led to the development of the cell theory. organelle Prokaryotic cells lack a nucleus and most internal structures of eukaryotic cells. prokaryotic cell eukaryotic cell Connect to Your World You and all other organisms are made of cells. As you saw on the previous page, > a cell’s structure is closely related to its function. Today we know that cells are the smallest unit of living matter that can carry out all processes required for life. But before the 1600s, people had many other ideas about the basis of life. Like many breakthroughs, the discovery of cells was aided by the development of new technology—in this case, the microscope. MAIN IDEA Early studies led to the development of the READING TOOLBOX cell theory. TAKING NOTES As you read, make an outline Almost all cells are too small to see without the aid of a microscope. Although using the headings as topics. glass lenses had been used to magnify images for hundreds of years, the early Summarize details that further lenses were not powerful enough to reveal individual cells. The invention of explain those ideas. the compound microscope in the late 1500s was an early step toward this I. Main Idea discovery. The Dutch eyeglass maker Zacharias Janssen, who was probably A. Supporting idea assisted by his father, Hans, usually gets credit for this invention. 1. Detail A compound microscope contains two or more lenses. Total magnification, the 2. -
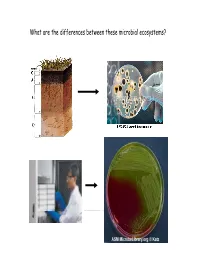
What Are the Differences Between These Microbial Ecosystems? Wwwwe Now Know That, Like Other Org Gm,Anisms, Bacteria Exhibit Social Behaviors
What are the differences between these microbial ecosystems? WWwwe now know that, like other org gm,anisms, bacteria exhibit social behaviors. Bacterial cell escaping for a rare moment of peace and quiet contemplation Sociomicrobiology I. Cell signaling A. Definition/description B. Intraspecific (within spp.): Myxococcus C. Interspecific (between spp.): Pseudomonas aureofaciens II. Biofilms A. Biofilm formation B. Planktonic cells vs. biofilm cells C. General characteristics, structures D. Biofilms as social entities Small diffusible molecules mediate bacterial communication O O N H AHL O O O N H O http://www.ted.com/index.php/talks/bonnie_bassler_on_how_bacteria_communicate.html Why cell-cell signaling in bacteria? Often, single cells in a population might benefit from knowing how many cells are present… “A multitude of bacteria are stronger than a few, thus by union are able overcome obstacles too great for few.” -- Dr. Erwin F. Smith, 1905 (Father of Plant Bacteriology) Pseudomonas aeruginosa in lungs Xylella fastidiosa in xylem Why cell-cell signaling in bacteria? Cell-cell signaling enables bacteria to coordinate behavior to respond quickly to environmental stimuli… such as: -presence of suitable host -change in nutrient availability -defense/competition against other microorganisms -many others! CmmiCommunica tion among btbacteri i:a: The example of Myxococcus xanthus, the Wolf Pack feeder of bacteria http://cmgm.stanford.edu/devbio/kaiserlab/about_myxo/about_myxococcus.html Cooperation among cells in a population: myxobacteria. The myxobacteria are Gram-negative, ubiquitous, soil-dwelling bacteria that are capable of multicellular, social behaviour. In the presence of nutrients, “swarms” of myxobacteria feed cooperatively by sharing extracellular digestive enzymes, and can prey on other bacteria. -

Structure of Bacterial and Archaeal Cells
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION 4 CHAPTER PREVIEW 4.1 There Is Tremendous Diversity Structure of Among the Bacteria and Archaea 4.2 Prokaryotes Can Be Distinguished by Their Cell Shape and Arrangements Bacterial and 4.3 An Overview to Bacterial and Archaeal Cell Structure 4.4 External Cell Structures Interact Archaeal Cells with the Environment Investigating the Microbial World 4: Our planet has always been in the “Age of Bacteria,” ever since the The Role of Pili first fossils—bacteria of course—were entombed in rocks more than TexTbook Case 4: An Outbreak of 3 billion years ago. On any possible, reasonable criterion, bacteria Enterobacter cloacae Associated with a Biofilm are—and always have been—the dominant forms of life on Earth. —Paleontologist Stephen J. Gould (1941–2002) 4.5 Most Bacterial and Archaeal Cells Have a Cell Envelope “Double, double toil and trouble; Fire burn, and cauldron bubble” is 4.6 The Cell Cytoplasm Is Packed the refrain repeated several times by the chanting witches in Shakespeare’s with Internal Structures Macbeth (Act IV, Scene 1). This image of a hot, boiling cauldron actu- MiCroinquiry 4: The Prokaryote/ ally describes the environment in which many bacterial, and especially Eukaryote Model archaeal, species happily grow! For example, some species can be iso- lated from hot springs or the hot, acidic mud pits of volcanic vents ( Figure 4.1 ). When the eminent evolutionary biologist and geologist Stephen J. Gould wrote the opening quote of this chapter, he, as well as most micro- biologists at the time, had no idea that embedded in these “bacteria” was another whole domain of organisms. -

Introduction to Microbiology
Introduction to microbiology Prof. dr hab. Beata M. Sobieszczańska Department of Microbiology University of Medicine • http://www.lekarski.umed.wroc.pl/mikrobiologia • schedules, rules, important information • Consulting hours – teachers are always available for students during consulting hours or classes – apart from consulting hours – you must chase ! • Sick leaves (original) must be shown to the teacher just after an absence but not longer than after two weeks otherwise a sick note will not be honored - a copy of the sick note must be delivered to the secretary office • Class tests – 10 open questions • Terms: 1st, 2nd – if failed commission test from the whole material at the end of semester • Students with the average 4.8 will be released from the final exam • Presence on lectures and classes are obligatory • The final grade from classes is the average of all grades during semester Your best friend in this year: Medical Microbiology by Patrick R. Murray, Ken S. Rosenthal, Michael A. Pfaller Answer questions: • Name important cell wall structures of GP and GN bacteria • What is a role of these structures in human diseases? • Name other than bacterial cell wall structures and explain their role in bacterial pathogenicity • Do you understand the term pathogenicity? • Name five different genera GP and GN bacteria and indicate the colour they have after Gram staining Answer questions: • Name clinically important bacteria producing endospores – why endospores are important? • What is the difference between capsule and glycocalyx layer on GP bacteria? • What is axial filament? What role it plays? What bacteria produce axial filaments? • Name two types of pili and their role in bacterial pathogenicity Most bacteria come in one of three basic shapes: coccus, rod or bacillus & spiral MURRAY 7th ed. -

Prokaryotes – Bacteria
Prokaryotes – Bacteria Prokaryotes, which includes, bacteria are the simplest of all the cells. All prokaryotes have a single, circular chromosome and lack a nucleus and membrane-bound organelles. There are two major groups of prokaryotic organisms --- the Kingdom Eubacteria and the Kingdom Archaebacteria. Eubacteria are known as true bacteria. They are the most common type of prokaryote. They are found everywhere, on surfaces and in the soil. Archaebacteria or the ancient bacteria are found in extreme environments, like hot sulfur springs and thermal vents in the ocean floor. They belong to the domain Archaea. Archaebacteria are thought to be some of the oldest life forms on earth. 1.What characteristics do all prokaryotes have in common? 2.What is the best known prokaryote and where can they be found? 3.Name the 2 kingdoms for prokaryotes. 4.Name the 2 bacterial domains. 1 5.Where are the bacterial members of the domain Archaea found? Give an example. 6.What are thought to be the oldest organisms on Earth? Most bacteria are heterotrophic and don't make their own food. That means they have to rely on other organisms to provide them with food. Some bacteria such as the cyanobacteria contain chlorophyll and can make their own food. These bacteria have to break down, or decompose, other living things to obtain energy. Very few bacteria cause illness. Some bacteria are used to make food, such as cheese and yogurt. Scientists have genetically engineered a type of bacteria that breaks down oil from oil spills. Some bacteria like E.coli, live inside the guts of animals and help them to digest food.