Characterization of Light-Enhanced Respiration in Cyanobacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Prokaryotes (Domains Bacteria & Archaea)
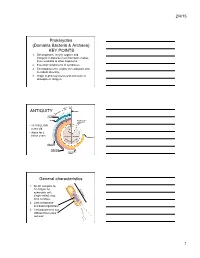
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Alternative Oxidase: a Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis During Abiotic and Biotic Stress in Plants
Int. J. Mol. Sci. 2013, 14, 6805-6847; doi:10.3390/ijms14046805 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants Greg C. Vanlerberghe Department of Biological Sciences and Department of Cell and Systems Biology, University of Toronto Scarborough, 1265 Military Trail, Toronto, ON, M1C1A4, Canada; E-Mail: [email protected]; Tel.: +1-416-208-2742; Fax: +1-416-287-7676 Received: 16 February 2013; in revised form: 8 March 2013 / Accepted: 12 March 2013 / Published: 26 March 2013 Abstract: Alternative oxidase (AOX) is a non-energy conserving terminal oxidase in the plant mitochondrial electron transport chain. While respiratory carbon oxidation pathways, electron transport, and ATP turnover are tightly coupled processes, AOX provides a means to relax this coupling, thus providing a degree of metabolic homeostasis to carbon and energy metabolism. Beside their role in primary metabolism, plant mitochondria also act as “signaling organelles”, able to influence processes such as nuclear gene expression. AOX activity can control the level of potential mitochondrial signaling molecules such as superoxide, nitric oxide and important redox couples. In this way, AOX also provides a degree of signaling homeostasis to the organelle. Evidence suggests that AOX function in metabolic and signaling homeostasis is particularly important during stress. These include abiotic stresses such as low temperature, drought, and nutrient deficiency, as well as biotic stresses such as bacterial infection. This review provides an introduction to the genetic and biochemical control of AOX respiration, as well as providing generalized examples of how AOX activity can provide metabolic and signaling homeostasis. -

The First Cells Were Most Likely Very Simple Prokaryotic Forms. Ra- Spirochetes
T HE O RIGIN OF E UKARYOTIC C ELLS The first cells were most likely very simple prokaryotic forms. Ra- spirochetes. Ingestion of prokaryotes that resembled present-day diometric dating indicates that the earth is 4 to 5 billion years old cyanobacteria could have led to the endosymbiotic development of and that prokaryotes may have arisen more than 3.5 billion years chloroplasts in plants. ago. Eukaryotes are thought to have first appeared about 1.5 billion Another hypothesis for the evolution of eukaryotic cells proposes years ago. that the prokaryotic cell membrane invaginated (folded inward) to en- The eukaryotic cell might have evolved when a large anaerobic close copies of its genetic material (figure 1b). This invagination re- (living without oxygen) amoeboid prokaryote ingested small aerobic (liv- sulted in the formation of several double-membrane-bound entities ing with oxygen) bacteria and stabilized them instead of digesting them. (organelles) in a single cell. These entities could then have evolved This idea is known as the endosymbiont hypothesis (figure 1a) and into the eukaryotic mitochondrion, nucleus, and chloroplasts. was first proposed by Lynn Margulis, a biologist at Boston Univer- Although the exact mechanism for the evolution of the eu- sity. (Symbiosis is an intimate association between two organisms karyotic cell will never be known with certainty, the emergence of of different species.) According to this hypothesis, the aerobic bac- the eukaryotic cell led to a dramatic increase in the complexity and teria developed into mitochondria, which are the sites of aerobic diversity of life-forms on the earth. At first, these newly formed eu- respiration and most energy conversion in eukaryotic cells. -

Prokaryotes, Protists, Reconstructing the Photosynthesis, Evolution of Living Things
Prokaryotes, Protists, Reconstructing the Photosynthesis, evolution of living things Endosymbiosis • Systematists study evolutionary relationships Ch 28 & 29 •Look for shared derived (=different from ancestor) traits to group organisms • Evidence used: morphology, 26 February 2009 development, and molecular data (especially DNA sequences) ECOL 182R UofA K. E. Bonine 1 2 Why can’t we figure it out perfectly? • More distant history is obscured by more changes trypanosomes • Among oldest lineages of Bacteria and Archaea in particular, lots of “lateral gene transfer.” Makes it difficult to infer relationships from phylogeny of red blood cells single genes. 3 4 Early prokaryote fossil Diversity of Prokaryotes Bacteria & Archaea 5 6 1 What are microbes? Life can be divided into 3 domains • Only a minority make us sick •Robert Koch, Germ Theory of Disease • In ordinary English, might be anything small 3.8bya –bacteria 1.5bya –yeast –protists – viruses •Prokaryotes = bacteria + archaea • In science, classify by evolutionary • Prokaryote was ancestral and only form for relationships… 7 billions of years 8 Eukarya Scheme has been revised before: Haeckel (1894) Whittaker (1959) Woese (1977) Woese (1990) Three kingdoms Five kingdoms Six kingdoms Three domains Eubacteria Bacteria Monera (prokaryotes) Protista Archaebacteria Archaea Protista Protista Fungi Fungi Plantae Eukarya are Prokaryotes monophyletic, Plantae Plantae Animalia Animalia Animalia paraphyleticparaphyletic, polyphyletic? 9 modified from Wikipedia 10 Shared by all 3 domains Unique to -

Prokaryote Vs. Eukaryote Campaign Biology Name: ______Period: ______
Prokaryote vs. Eukaryote Campaign Biology Name: ___________________________________________ Period: ____________ Prokaryotes vs. Eukaryotes: A Campaign for Election as Coolest Cell Type! Purpose: While prokaryotes and eukaryotes share some similar structures and characteristics of life, the two lead some pretty different lives! Your job in this assignment is to create a campaign poster and a campaign platform commercial for a prokaryote or a eukaryote as they campaign to be elected coolest cell type! Directions: Each group will be assigned a campaign platform from those listed below. Use the resources listed (as well as any others you might find) to understand why your platform makes your cell type the coolest. Create a campaign poster (using one slide on a Google Presentation, shared between partners, and submitted by due date via a form) and a short campaign platform commercial (given as a speech in class) using the attached rubric. Prokaryotic campaign platforms: 1. Ability to form endospores http://goo.gl/Y1tUK http://goo.gl/DhVNe 2. Ability to form biofilms with other cells http://goo.gl/umy7Z http://goo.gl/m9d5W 3. Quorum sensing neighboring cells http://goo.gl/DSiJU http://goo.gl/IFFtA 4. Production of bacteriocins http://goo.gl/lW75y http://goo.gl/WzFv1 5. Endosymbiotic theory http://goo.gl/6VIba http://goo.gl/uSYh4 Eukaryotic campaign platforms: 6. Contain organelles that have their own genetic material in addition to that found in the nucleus http://goo.gl/6VIba http://goo.gl/Nqesq 7. Vesicles http://goo.gl/bwF3x http://goo.gl/j3qY3 8. Membrane bound organelles that facilitate transport (endomembrane system) http://goo.gl/j3qY3 http://goo.gl/i4brZ 9. -

Evidence for Extensive Heterotrophic Metabolism, Antioxidant Action, and Associated Regulatory Events During Winter Hardening In
Collakova et al. BMC Plant Biology 2013, 13:72 http://www.biomedcentral.com/1471-2229/13/72 RESEARCH ARTICLE Open Access Evidence for extensive heterotrophic metabolism, antioxidant action, and associated regulatory events during winter hardening in Sitka spruce Eva Collakova1, Curtis Klumas2, Haktan Suren2,3,ElijahMyers2,LenwoodSHeath4, Jason A Holliday3 and Ruth Grene1* Abstract Background: Cold acclimation in woody perennials is a metabolically intensive process, but coincides with environmental conditions that are not conducive to the generation of energy through photosynthesis. While the negative effects of low temperatures on the photosynthetic apparatus during winter have been well studied, less is known about how this is reflected at the level of gene and metabolite expression, nor how the plant generates primary metabolites needed for adaptive processes during autumn. Results: The MapMan tool revealed enrichment of the expression of genes related to mitochondrial function, antioxidant and associated regulatory activity, while changes in metabolite levels over the time course were consistent with the gene expression patterns observed. Genes related to thylakoid function were down-regulated as expected, with the exception of plastid targeted specific antioxidant gene products such as thylakoid-bound ascorbate peroxidase, components of the reactive oxygen species scavenging cycle, and the plastid terminal oxidase. In contrast, the conventional and alternative mitochondrial electron transport chains, the tricarboxylic acid cycle, and redox-associated proteins providing reactive oxygen species scavenging generated by electron transport chains functioning at low temperatures were all active. Conclusions: A regulatory mechanism linking thylakoid-bound ascorbate peroxidase action with “chloroplast dormancy” is proposed. Most importantly, the energy and substrates required for the substantial metabolic remodeling that is a hallmark of freezing acclimation could be provided by heterotrophic metabolism. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -

Cell Theory VOCABULARY Unit 2: Cells Unit 2: > RE B
3.1 Cell Theory KEY CONCEPT Cells are the basic unit of life. VOCABULARY cell theory MAIN IDEAS cytoplasm Early studies led to the development of the cell theory. organelle Prokaryotic cells lack a nucleus and most internal structures of eukaryotic cells. prokaryotic cell eukaryotic cell Connect to Your World You and all other organisms are made of cells. As you saw on the previous page, > a cell’s structure is closely related to its function. Today we know that cells are the smallest unit of living matter that can carry out all processes required for life. But before the 1600s, people had many other ideas about the basis of life. Like many breakthroughs, the discovery of cells was aided by the development of new technology—in this case, the microscope. MAIN IDEA Early studies led to the development of the READING TOOLBOX cell theory. TAKING NOTES As you read, make an outline Almost all cells are too small to see without the aid of a microscope. Although using the headings as topics. glass lenses had been used to magnify images for hundreds of years, the early Summarize details that further lenses were not powerful enough to reveal individual cells. The invention of explain those ideas. the compound microscope in the late 1500s was an early step toward this I. Main Idea discovery. The Dutch eyeglass maker Zacharias Janssen, who was probably A. Supporting idea assisted by his father, Hans, usually gets credit for this invention. 1. Detail A compound microscope contains two or more lenses. Total magnification, the 2. -

Structure of Bacterial and Archaeal Cells
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION 4 CHAPTER PREVIEW 4.1 There Is Tremendous Diversity Structure of Among the Bacteria and Archaea 4.2 Prokaryotes Can Be Distinguished by Their Cell Shape and Arrangements Bacterial and 4.3 An Overview to Bacterial and Archaeal Cell Structure 4.4 External Cell Structures Interact Archaeal Cells with the Environment Investigating the Microbial World 4: Our planet has always been in the “Age of Bacteria,” ever since the The Role of Pili first fossils—bacteria of course—were entombed in rocks more than TexTbook Case 4: An Outbreak of 3 billion years ago. On any possible, reasonable criterion, bacteria Enterobacter cloacae Associated with a Biofilm are—and always have been—the dominant forms of life on Earth. —Paleontologist Stephen J. Gould (1941–2002) 4.5 Most Bacterial and Archaeal Cells Have a Cell Envelope “Double, double toil and trouble; Fire burn, and cauldron bubble” is 4.6 The Cell Cytoplasm Is Packed the refrain repeated several times by the chanting witches in Shakespeare’s with Internal Structures Macbeth (Act IV, Scene 1). This image of a hot, boiling cauldron actu- MiCroinquiry 4: The Prokaryote/ ally describes the environment in which many bacterial, and especially Eukaryote Model archaeal, species happily grow! For example, some species can be iso- lated from hot springs or the hot, acidic mud pits of volcanic vents ( Figure 4.1 ). When the eminent evolutionary biologist and geologist Stephen J. Gould wrote the opening quote of this chapter, he, as well as most micro- biologists at the time, had no idea that embedded in these “bacteria” was another whole domain of organisms. -

Coversheet for Thesis in Sussex Research Online
A University of Sussex DPhil thesis Available online via Sussex Research Online: http://sro.sussex.ac.uk/ This thesis is protected by copyright which belongs to the author. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Please visit Sussex Research Online for more information and further details Structure, function and mechanism of the alternative oxidases Luke Young September 2013 Submitted in partial fulfilment towards the requirements for the degree of Doctor of Philosophy (DPhil) i | P a g e I hereby declare that this thesis has not been and will not be, submitted in whole or in part to another University for the award of any other degree. Signature ............................................. ii | P a g e University of Sussex Luke Young Submitted for the degree of Doctor of philosophy Structure function and mechanism of the alternative oxidases Summary The alternative oxidase (AOX) is the terminal protein in the alternative oxidation pathway found in plants, fungi and some protozoa. One of the more prominent protozoa that contain AOX within the bloodstream form is Trypanosoma brucei, the causative agent of human African trypanosomiasis (HAT), in which the parasite has demonstrated to be totally dependent upon the protein for continued respiration. Given the lack of AOX in mammalian cells, the protein represents an attractive chemotherapeutic target for trypanosidal activity. -

Prokaryotes – Bacteria
Prokaryotes – Bacteria Prokaryotes, which includes, bacteria are the simplest of all the cells. All prokaryotes have a single, circular chromosome and lack a nucleus and membrane-bound organelles. There are two major groups of prokaryotic organisms --- the Kingdom Eubacteria and the Kingdom Archaebacteria. Eubacteria are known as true bacteria. They are the most common type of prokaryote. They are found everywhere, on surfaces and in the soil. Archaebacteria or the ancient bacteria are found in extreme environments, like hot sulfur springs and thermal vents in the ocean floor. They belong to the domain Archaea. Archaebacteria are thought to be some of the oldest life forms on earth. 1.What characteristics do all prokaryotes have in common? 2.What is the best known prokaryote and where can they be found? 3.Name the 2 kingdoms for prokaryotes. 4.Name the 2 bacterial domains. 1 5.Where are the bacterial members of the domain Archaea found? Give an example. 6.What are thought to be the oldest organisms on Earth? Most bacteria are heterotrophic and don't make their own food. That means they have to rely on other organisms to provide them with food. Some bacteria such as the cyanobacteria contain chlorophyll and can make their own food. These bacteria have to break down, or decompose, other living things to obtain energy. Very few bacteria cause illness. Some bacteria are used to make food, such as cheese and yogurt. Scientists have genetically engineered a type of bacteria that breaks down oil from oil spills. Some bacteria like E.coli, live inside the guts of animals and help them to digest food. -

Prokaryotic Growth and Nutrition
37623_CH05_137_160.pdf 11/11/06 7:23 AM Page 137 © Jones and Bartlett Publishers. NOT FOR SALE OR DISTRIBUTION 5 Chapter Preview and Key Concepts 5.1 Prokaryotic Reproduction Prokaryotic • Binary fission produces genetically-identical daughter cells. • Prokaryotes vary in their generation times. Growth and 5.2 Prokaryotic Growth • Bacterial population growth goes through four phases. • Endospores are dormant structures to endure Nutrition times of nutrient stress. • Growth of prokaryotic populations is sensitive to temperature, oxygen gas, and pH. But who shall dwell in these worlds if they be inhabited? . Are we or 5.3 Culture Media and Growth Measurements • Culture media contain the nutrients needed for they Lords of the World? . optimal prokaryotic growth. —Johannes Kepler (quoted in The Anatomy of Melancholy) • Special chemical formulations can be devised to isolate and identify some prokaryotes. MicroInquiry 5: Identification of Bacterial Species Books have been written about it; movies have been made; even a • Two standard methods are available to produce radio play in 1938 about it frightened thousands of Americans. What pure cultures. is it? Martian life. In 1877 the Italian astronomer, Giovanni Schiapar- • Prokaryotic growth can be measured by direct elli, saw lines on Mars, which he and others assumed were canals built and indirect methods. by intelligent beings. It wasn’t until well into the 20th century that this notion was disproved. Still, when we gaze at the red planet, we won- der: Did life ever exist there? We are not the only ones wondering. Astronomers, geologists, and many other scientists have asked the same question. Today microbiol- ogists have joined their comrades, wondering if microbial life once existed on the Red Planet or, for that matter, elsewhere in our Solar System.