M220 Lecture 5 Discuss Principle Differences
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

G:\CLASSES\BI 345N6\Bi345n6 W07\Biol 345 W07
BIOLOGY 345 Name _____________________ Midterm I - 05 February 2007 PART I. Multiple choice questions – (4 points each, 36 points total). 1. Which of the following metals was used in the construction of pipes in early Rome and may have contributed to the fall of the Roman emprire? A. Iron B. Bronze C. Gold D. Lead E. Silver 2. Louis Pasteur is recognized as the scientist who finally refuted which hypothesis using experiments involving microorganisms and swan-necked flasks? A. Germ Theory B. Spontaneous generation C. Natural selection D. Ontogeny recapitulates phylogeny E. Pasteurization principle 3. Cell walls are important features in both bacteria and archaea. Which of the following componds best describes the biomolecular subunits one might find exclusively in an archaeal cell wall? A. Diaminopimelic acid (DAP) & D-alanine interbridge B. L-lysine & pentaglycine interbridge C. N-acetylglucosamine (NAG) & N-acetylmuramic acid (NAM) glycan D. N-acetylglucosamine (NAG) & N-acetyltalosaminuronic acid (NAT) glycan E. Dipicolinic acid & Ca++ 4. Considering the multitude of potential metabolic processes available to prokaryotes, which of the following are used to describe specific types of chemotrophic metabolisms? A. Energy source B. Carbon source C. Electron source D. Hydrogen source E. Electron acceptor Page 1 of 8 5. The majority of the bacterial cell’s dry weight (96.1% in E. coli) is due to just a few macromolecules and polymers. Which of the following is NOT a major component of a bacterial cell? A. RNA B. Peptidoglycan (aka murein) C. Proteins D. Vitamins E. Lipids 6. Which of the following is an invariant feature found among all microbial cells? A. -

Antonie Van Leeuwenhoek Journal of Microbiology
Antonie van Leeuwenhoek Journal of Microbiology Kroppenstedtia pulmonis sp. nov. and Kroppenstedtia sanguinis sp. nov., isolated from human patients --Manuscript Draft-- Manuscript Number: ANTO-D-15-00548R1 Full Title: Kroppenstedtia pulmonis sp. nov. and Kroppenstedtia sanguinis sp. nov., isolated from human patients Article Type: Original Article Keywords: Kroppenstedtia species, Kroppenstedtia pulmonis, Kroppenstedtia sanguinis, polyphasic taxonomy, 16S rRNA gene, thermoactinomycetes Corresponding Author: Melissa E Bell, MS Centers for Disease Control and Prevention Atlanta, Georgia UNITED STATES Corresponding Author Secondary Information: Corresponding Author's Institution: Centers for Disease Control and Prevention Corresponding Author's Secondary Institution: First Author: Melissa E Bell, MS First Author Secondary Information: Order of Authors: Melissa E Bell, MS Brent A. Lasker, PhD Hans-Peter Klenk, PhD Lesley Hoyles, PhD Catherine Spröer Peter Schumann June Brown Order of Authors Secondary Information: Funding Information: Abstract: Three human clinical strains (W9323T, X0209T and X0394) isolated from lung biopsy, blood and cerebral spinal fluid, respectively, were characterized using a polyphasic taxonomic approach. Comparative analysis of the 16S rRNA gene sequences showed the three strains belonged to two novel branches within the genus Kroppenstedtia: 16S rRNA gene sequence analysis of W9323T showed closest sequence similarity to Kroppenstedtia eburnea JFMB-ATE T (95.3 %), Kroppenstedtia guangzhouensis GD02T (94.7 %) and strain X0209T (94.6 %); sequence analysis of strain X0209T showed closest sequence similarity to K. eburnea JFMB-ATE T (96.4 %) and K. guangzhouensis GD02T (96.0 %). Strains X0209T and X0394 were 99.9 % similar to each other by 16S rRNA gene sequence analysis. The DNA-DNA relatedness was 94.6 %, confirming that X0209T and X0394 belong to the same species. -

Infection Control
infection control JANICE CARR The above photo depicts an E.coli (ATCC 11775) biofilm grown on PC (polycarbonate) coupons using a CDC biofilm reactor. Microorganisms often colonize, and adhere strongly to living and non-living surfaces forming biofilms, and at times, demonstrate an increased resistance to antimicrobials. Biofilms on indwelling medical devices pose a serious threat to public health. BIOFILMS:BIOFILMS: Friend or Foe? By NICOLE KENNY, B.Sc, Assoc.Chem., Director of Professional & Technical Services, Virox Technologies Inc 38 Sanitation Canada - SEPTEMBER / OCTOBER 2006 JANICE CARR Scanning electron micrograph of a Staphylococcus biofilm on the inner surface of a needleless connector. A distinguishing characteristic of biofilms is the presence of extracellular polymeric substances, primarily polysaccharides, surrounding and encasing the cells. Here, there polysaccharides have been visualized by scanning electron microscopy. Picture yourself a con- “Why didn’t I listen to my mother and take Biofilms can be dangerous or benefi- testant on Jeopardy. Alex more science courses?” But in that split cial depending on where they are found Tribec has just asked you second you also remember a documen- and of which organisms they are com- to choose the category. tary you watched on CNN about whirl- prised. In industry, biofilms are responsi- You’re lagging behind the pool tubs and you know the answer. ble for billions of dollars in lost produc- leader by $400. All but “Alex, what are BIOFILMS?” tivity due to equipment damage, notori- one of the $500 questions ously famous for causing pipes to plug or Phave been taken, and the last category has THE ISSUE corrode. -

Functional Anatomy of Prokaryotes and Eukaryotes
FUNCTIONAL ANATOMY OF PROKARYOTES AND EUKARYOTES BY DR JAWAD NAZIR ASSISTANT PROFESSOR DEPARTMENT OF MICROBIOLOGY UNIVERSITY OF VETERINARY AND ANIMAL SCIENCES, LAHORE Prokaryotes vs Eukaryotes Prokaryote comes from the Greek words for pre-nucleus Eukaryote comes from the Greek words for true nucleus. Functional anatomy of prokaryotes Prokaryotes vs Eukaryotes Prokaryotes Eukaryotes One circular chromosome, not in Paired chromosomes, in nuclear a membrane membrane No histones Histones No organelles Organelles Peptidoglycan cell walls Polysaccharide cell walls Binary fission Mitotic spindle Functional anatomy of prokaryotes Size and shape Average size: 0.2 -1.0 µm 2 - 8 µm Basic shapes: Functional anatomy of prokaryotes Size and shape Pairs: diplococci, diplobacilli Clusters: staphylococci Chains: streptococci, streptobacilli Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Unusual shapes Star-shaped Stella Square Haloarcula Most bacteria are monomorphic A few are pleomorphic Genus: Stella Genus: Haloarcula Functional anatomy of prokaryotes Bacterial cell structure Structures external to cell wall Cell wall itself Structures internal to cell wall Functional anatomy of prokaryotes Glycocalyx Outside cell wall Usually sticky A capsule is neatly organized A slime layer is unorganized & loose Extracellular polysaccharide allows cell to attach Capsules prevent phagocytosis Association with diseases B. anthracis S. pneumoniae Functional anatomy of prokaryotes Flagella Outside cell wall Filament made of chains of flagellin Attached to a protein hook Anchored to the wall and membrane by the basal body Functional anatomy of prokaryotes Flagella Arrangement Functional anatomy of prokaryotes Bacterial motility Rotate flagella to run or tumble Move toward or away from stimuli (taxis) Flagella proteins are H antigens (e.g., E. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -
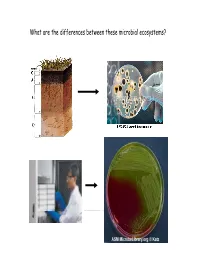
What Are the Differences Between These Microbial Ecosystems? Wwwwe Now Know That, Like Other Org Gm,Anisms, Bacteria Exhibit Social Behaviors
What are the differences between these microbial ecosystems? WWwwe now know that, like other org gm,anisms, bacteria exhibit social behaviors. Bacterial cell escaping for a rare moment of peace and quiet contemplation Sociomicrobiology I. Cell signaling A. Definition/description B. Intraspecific (within spp.): Myxococcus C. Interspecific (between spp.): Pseudomonas aureofaciens II. Biofilms A. Biofilm formation B. Planktonic cells vs. biofilm cells C. General characteristics, structures D. Biofilms as social entities Small diffusible molecules mediate bacterial communication O O N H AHL O O O N H O http://www.ted.com/index.php/talks/bonnie_bassler_on_how_bacteria_communicate.html Why cell-cell signaling in bacteria? Often, single cells in a population might benefit from knowing how many cells are present… “A multitude of bacteria are stronger than a few, thus by union are able overcome obstacles too great for few.” -- Dr. Erwin F. Smith, 1905 (Father of Plant Bacteriology) Pseudomonas aeruginosa in lungs Xylella fastidiosa in xylem Why cell-cell signaling in bacteria? Cell-cell signaling enables bacteria to coordinate behavior to respond quickly to environmental stimuli… such as: -presence of suitable host -change in nutrient availability -defense/competition against other microorganisms -many others! CmmiCommunica tion among btbacteri i:a: The example of Myxococcus xanthus, the Wolf Pack feeder of bacteria http://cmgm.stanford.edu/devbio/kaiserlab/about_myxo/about_myxococcus.html Cooperation among cells in a population: myxobacteria. The myxobacteria are Gram-negative, ubiquitous, soil-dwelling bacteria that are capable of multicellular, social behaviour. In the presence of nutrients, “swarms” of myxobacteria feed cooperatively by sharing extracellular digestive enzymes, and can prey on other bacteria. -

Verrucosispora Rhizosphaerae Sp. Nov., Isolated from Mangrove Rhizosphere Soil
Antonie van Leeuwenhoek DOI 10.1007/s10482-017-0933-4 ORIGINAL PAPER Verrucosispora rhizosphaerae sp. nov., isolated from mangrove rhizosphere soil Qing-yi Xie . Xiao-dong Bao . Qing-yu Ma . Fan-dong Kong . Man-li Zhou . Bing Yan . You-xing Zhao Received: 24 May 2017 / Accepted: 19 August 2017 Ó The Author(s) 2017. This article is an open access publication Abstract An actinomycete strain, 2603PH03T,was phosphatidylserine and an unidentified phospholipid. isolated from a mangrove rhizosphere soil sample The DNA G?C content was determined to be collected in Wenchang, China. Phylogenetic analysis of 70.1 mol%. The results of physiological and biochem- the 16S rRNA gene sequence of strain 2603PH03T ical tests and low DNA-DNA relatedness readily indicated high similarity to Verrucosispora gifthornen- distinguished the isolate from the closely related sis DSM 44337T (99.4%), Verrucosispora andamanen- species. On the basis of these phenotypic and genotypic sis (99.4%), Verrucosispora fiedleri MG-37T (99.4%) data, strain 2603PH03T is concluded to represent a novel and Verrucosispora maris AB18-032T (99.4%). The species of the genus Verrucosispora, for which the name cell wall was found to contain meso-diaminopimelic Verrucosispora rhizosphaerae sp. nov. is proposed. The acid and glycine. The major menaquinones were type strain is 2603PH03T (=CCTCC AA T T identified as MK-9(H4), MK-9(H6) and MK-9(H8), 2016023 = DSM 45673 ). with MK-9(H2), MK-10(H2), MK-9(H10) and MK- 10(H6) as minor components. The characteristic whole Keywords Verrucosispora rhizosphaerae sp. nov. Á cell sugars were found to be xylose and mannose. -

Trans-Kingdom Enzyme Shared by Chlamydia and Plants for Synthesis of Diaminopimelate͞lysine
L,L-diaminopimelate aminotransferase, a trans-kingdom enzyme shared by Chlamydia and plants for synthesis of diaminopimelate͞lysine Andrea J. McCoy*, Nancy E. Adams*, Andre´O. Hudson†, Charles Gilvarg‡, Thomas Leustek†, and Anthony T. Maurelli*§ *Department of Microbiology and Immunology, F Edward He´bert School of Medicine, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814-4799; †Biotech Center and Department of Plant Biology and Pathology, Rutgers University, 59 Dudley Road, New Brunswick, NJ 08901-8520; and ‡Department of Molecular Biology, Princeton University, Princeton, NJ 08544 Communicated by Roy Curtiss, Arizona State University, Tempe, AZ, October 2, 2006 (received for review July 15, 2006) The synthesis of meso-diaminopimelic acid (m-DAP) in bacteria is we refer to the four-step synthesis of THDP as the upper m-DAP essential for both peptidoglycan and lysine biosynthesis. From synthesis pathway. From THDP, three variant pathways have genome sequencing data, it was unclear how bacteria of the been defined for m-DAP synthesis in the bacteria: the succin Chlamydiales order would synthesize m-DAP in the absence of ylase, acetylase, and dehydrogenase pathways. The succinylase dapD, dapC, and dapE, which are missing from the genome. Here, pathway uses succinylated intermediates and is the most widely we assessed the biochemical capacity of Chlamydia trachomatis distributed in bacteria. Genes encoding THDP succinyltrans- serovar L2 to synthesize m-DAP. Expression of the chlamydial asd, ferase (dapD) and N-succinyl-L,L-DAP desuccinylase (dapE) dapB, and dapF genes in the respective Escherichia coli m-DAP have been characterized, whereas two enzymes, DapC and auxotrophic mutants restored the mutants to DAP prototrophy. -

Introduction to Microbiology
Introduction to microbiology Prof. dr hab. Beata M. Sobieszczańska Department of Microbiology University of Medicine • http://www.lekarski.umed.wroc.pl/mikrobiologia • schedules, rules, important information • Consulting hours – teachers are always available for students during consulting hours or classes – apart from consulting hours – you must chase ! • Sick leaves (original) must be shown to the teacher just after an absence but not longer than after two weeks otherwise a sick note will not be honored - a copy of the sick note must be delivered to the secretary office • Class tests – 10 open questions • Terms: 1st, 2nd – if failed commission test from the whole material at the end of semester • Students with the average 4.8 will be released from the final exam • Presence on lectures and classes are obligatory • The final grade from classes is the average of all grades during semester Your best friend in this year: Medical Microbiology by Patrick R. Murray, Ken S. Rosenthal, Michael A. Pfaller Answer questions: • Name important cell wall structures of GP and GN bacteria • What is a role of these structures in human diseases? • Name other than bacterial cell wall structures and explain their role in bacterial pathogenicity • Do you understand the term pathogenicity? • Name five different genera GP and GN bacteria and indicate the colour they have after Gram staining Answer questions: • Name clinically important bacteria producing endospores – why endospores are important? • What is the difference between capsule and glycocalyx layer on GP bacteria? • What is axial filament? What role it plays? What bacteria produce axial filaments? • Name two types of pili and their role in bacterial pathogenicity Most bacteria come in one of three basic shapes: coccus, rod or bacillus & spiral MURRAY 7th ed. -

Actinoplanes Aureus Sp. Nov., a Novel Protease- Producing Actinobacterium Isolated from Soil
Actinoplanes aureus sp. nov., a novel protease- producing actinobacterium isolated from soil Xiujun Sun Northeast Agricultural University Xianxian Luo Northeast Agricultural University Chuan He Northeast Agricultural University Zhenzhen Huang Northeast Agricultural University Junwei Zhao Northeast Agricultural University Beiru He Northeast Agricultural University Xiaowen Du Northeast Agricultural University Wensheng Xiang Northeast Agricultural University Jia Song ( [email protected] ) Northeast Agricultural University https://orcid.org/0000-0002-0398-2666 Xiangjing Wang Northeast Agricultural University Research Article Keywords: Actinoplanes aureus sp. nov, genome, polyphasic analysis, 16S rRNA gene Posted Date: April 26th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-260966/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/19 Version of Record: A version of this preprint was published at Antonie van Leeuwenhoek on July 29th, 2021. See the published version at https://doi.org/10.1007/s10482-021-01617-4. Page 2/19 Abstract A novel protease-producing actinobacterium, designated strain NEAU-A11T, was isolated from soil collected from Aohan banner, Chifeng, Inner Mongolia Autonomous Region, China, and characterised using a polyphasic approach. On the basis of 16S rRNA gene sequence analysis, strain NEAU-A11T was indicated to belong to the genus Actinoplanes and was most closely related to Actinoplanes rectilineatus JCM 3194T (98.9 %). Cell walls contained meso-diaminopimelic acid as the diagnostic diamino acid and the whole-cell sugars were arabinose, xylose and glucose. The phospholipid prole contained diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol and two phosphatidylinositol mannosides. The predominant menaquinones were MK-9(H4), MK-9(H6) and MK- 9(H8). -

Name of the Manuscript
Available online: May 27, 2019 Commun.Fac.Sci.Univ.Ank.Series C Volume 28, Number 1, Pages 78-90 (2019) ISSN 1303-6025 E-ISSN 2651-3749 https://dergipark.org.tr/tr/pub/communc/issue/45050/570542 CHEMOTAXONOMY IN BACTERIAL SYSTEMATICS F. SEYMA GOKDEMİR, SUMER ARAS ABSTRACT. In taxonomy, polyphasic approach is based on the principle of combining and evaluating different types of data obtained from microorganisms. While, during characterization and identification of a microorganism, in the direction of polyphasic studies, chemotaxonomic analysis has of paramount importance for the determination of the most important differences between the family, genus and species comparatively. It is beyond doubt that, in recent years significant developments have been achieved in systematics by the aid of molecular biological studies. Phylogenetic data have revealed the hierarchical arrangement of the kinship relations between the given bacteria, however, this information cannot provide reliable data on the level of genus. At this stage, chemical markers play an important role in regulating inter-taxa relationships. Chemotaxonomy; is the whole of the characterizations made by using the similarities and differences of the biochemical properties of bacteria. In bacterial systematics, chemotaxonomy examines biochemical markers such as: amino acids and peptides (peptidoglycan), lipids (fatty acid, lipopolysaccharides, micolic acid and polar lipids), polysaccharides and related polymers (teicoic acid, whole sugar) and other complex polymeric compounds to find the distribution of members of different taxa and all of this information is used for classification and identification. In this review, how the chemotaxonomic data can be used in bacterial systematics and reflected to application within the field questions were evaluated.-REVIEW. -
Gram Negative Bacterial Structure Explain the Medical Implications of Spore Formation
Lecture (3) Bacterial cell structure Objectives Enumerate Essential and non-essential bacterial cell components Describe the common anatomical structures found in bacteria and explain their function [flagella, pili, glycocalyx, capsule, endospores, cytoplasm, inclusions, chromosome, plasmids, cell membrane, and cell wall]. Compare gram positive and gram negative bacterial structure Explain the medical implications of spore formation. Bacterial cell structure •Cell wall Essential •Cytoplasmic Membrane •Cytoplasm Structures •Nuclear body •Capsule. Non Essential •Flagella •Pili structures •Inclusion granules Cell wall Cytoplasmic Essential structures membrane Any bacterial cell is composed of the following structures (Essential structures): 1. Cell wall. 2. Cytoplasmic membrane. 3. Cytoplasm. 4. Nuclear body. Nuceloid Cytoplasm Non Essential Structures Capsule Inclusion Some (Not all) bacteria may granules contain one or more of the following structures: 1. Capsule. 2. Flagella (single Flagellum) 3. Fimbria (pili). Pili 4. Inclusion granules Flagellum Cell wall Cytoplasmic THE CELL WALL membrane The cell wall is a rigid structure that surrounds the bacterial cell just outside of the plasma membrane. Nuceloid Cytoplasm Cell wall Structure Bacteria are classified according to their cell wall as: Gram positive or Gram negative. Peptidoglycan Polysaccharide chains The main structural component of the cell wall. Peptidoglycan is formed of carbohydrate + protein. It consists of long polysaccharide chains that are cross-linked by amino acid bridges. Gram positive Cell Wall In Gram-positive bacteria the peptidoglycan forms a thick layer external to the cell membrane. Cell wall of gram positive bacteria also contain Teichoic acid molecules. Gram negative Cell Wall In Gram-negative bacteria, the peptidoglycan layer is thin and is overlaid by an outer membrane.