Year 9 BASKETBALL Knowledge Organiser Referee Signals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Development and Character of the Nazi Political Machine, 1928-1930, and the Isdap Electoral Breakthrough
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1976 The evelopmeD nt and Character of the Nazi Political Machine, 1928-1930, and the Nsdap Electoral Breakthrough. Thomas Wiles Arafe Jr Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Arafe, Thomas Wiles Jr, "The eD velopment and Character of the Nazi Political Machine, 1928-1930, and the Nsdap Electoral Breakthrough." (1976). LSU Historical Dissertations and Theses. 2909. https://digitalcommons.lsu.edu/gradschool_disstheses/2909 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. « The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing pega(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Year 9 Grammar Stream Knowledge Organiser 2020
Year 9 – Grammar Stream Knowledge Organisers Term 3 Swindon Academy 2020-21 Name: Tutor Group: Tutor & Room: “If you are not willing to learn, no one can help you. If you are determined to learn, no one can stop you.” Using your Knowledge Organiser and Quizzable Knowledge Organiser Knowledge Organisers Quizzable Knowledge Expectations for Prep and for Organisers using your Knowledge Organisers 1. Complete all prep work set in your subject prep book. 2. Bring your prep book to every lesson and ensure that you have completed all work by the deadline. 3. Take pride in your prep book – keep it neat and tidy. 4. Present work in your prep book to the same standard you are expected to do in class. Knowledge Organisers contain the These are designed to help you quiz essential knowledge that you MUST yourself on the essential Knowledge. know in order to be successful this year 5. Ensure that your use of SPAG is accurate. and in all subsequent years. 6. Write in blue or black pen and sketch in pencil. Use them to test yourself or get They will help you learn, revise and someone else to test you, until you 7. Ensure every piece of work has a title and date. retain what you have learnt in lessons are confident you can recall the in order to move the knowledge from information from memory. your short-term memory to long- 8. Use a ruler for straight lines. term memory. 9. If you are unsure about the prep, speak to your teacher. Top Tip Don’t write on your Quizzable Knowledge Organisers! 10. -

Paper 3 Weimar and Nazi Germany Revision Guide and Student Activity Book
Paper 3 Weimar and Nazi Germany Revision Guide and Student Activity Book Section 1 – Weimar Republic 1919-1929 What was Germany like before and after the First World War? Before the war After the war The Germans were a proud people. The proud German army was defeated. Their Kaiser, a virtual dictator, was celebrated for his achievements. The Kaiser had abdicated (stood down). The army was probably the finest in the world German people were surviving on turnips and bread (mixed with sawdust). They had a strong economy with prospering businesses and a well-educated, well-fed A flu epidemic was sweeping the country, killing workforce. thousands of people already weakened by rations. Germany was a superpower, being ruled by a Germany declared a republic, a new government dictatorship. based around the idea of democracy. The first leader of this republic was Ebert. His job was to lead a temporary government to create a new CONSTITUTION (SET OF RULES ON HOW TO RUN A COUNTRY) Exam Practice - Give two things you can infer from Source A about how well Germany was being governed in November 1918. (4 marks) From the papers of Jan Smuts, a South African politician who visited Germany in 1918 “… mother-land of our civilisation (Germany) lies in ruins, exhausted by the most terrible struggle in history, with its peoples broke, starving, despairing, from sheer nervous exhaustion, mechanically struggling forward along the paths of anarchy (disorder with no strong authority) and war.” Inference 1: Details in the source that back this up: Inference 2: Details in the source that back this up: On the 11th November, Ebert and the new republic signed the armistice. -
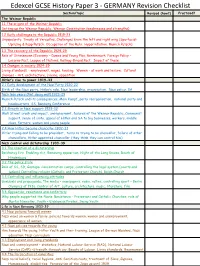
Edexcel GCSE History Paper 3 - GERMANY Revision Checklist Section/Topic Revised (How?) Practised? the Weimar Republic 1.1 the Origins of the Weimar Republic
Edexcel GCSE History Paper 3 - GERMANY Revision Checklist Section/topic Revised (how?) Practised? The Weimar Republic 1.1 The origins of the Weimar Republic Setting up the Weimar Republic, Weimar Constitution (weaknesses and strengths) 1.2 Early challenges to the Republic 1919-23 Unpopularity, Treaty of Versailles, Challenges from the left and right wing (Spartacist Uprising & Kapp Putsch, Occupation of the Ruhr, Hyperinflation, Munich Putsch) 1.3 The recovery of the Republic 1924-29 Role of Stresemann (Economy – Dawes and Young Plan, Rentenmark. Foreign Policy – Locarno Pact, League of Nations, Kellogg-Briand Pact. Impact of these. 1.4 Changes in society 1924-29 Living standards – employment, wages, housing. Women – at work and leisure. Cultural changes – Art, architecture, cinema, opposition Hitler’s rise to power 1919-33 2.1 Early development of the Nazi Party 1920-22 Birth of the Nazi party, Hitler’s role, Nazi leadership, organisation, Nazi policy, SA Nazi lean years (Not doing well) 1923-29 Munich Putsch and its consequences, Mein Kampf, party reorganisation, national party and headquarters, SS, Bamberg Conference 2.3 Growth in Nazi support 1929-32 Wall Street crash and impact, unemployment, failures of the Weimar Republic, Communist support, sense of unity, appeal of Hitler and SA to big businesses, workers, middle class, farmers, women and young people 2.4 How Hitler became chancellor 1932-33 Hitler trying and failing to be president, turns to trying to be chancellor, failure of other chancellors, Hitler appointed chancellor (they think they can control him) Nazi control and dictatorship 1933-39 3.1 The creation of a dictatorship Reichstag fire. -

The Evolution of Adolf Hitler's Weltanschuung
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. THE EVOLUTION OF ADOLF HITLER'S WELTANSCHAUUNG: A CRITICAL STUDY OF HIS RHETORIC, 1920 - 1926 A thesis presented in fulfilment of the requirements for the degree of Master of Arts at Massey University CAROLYN READ MASSEY UNIVERSITY 1997 For Max ACKNOWLEDGEMENTS This year has been a learning experience, especially in respect of self motivation, but I made it! I would like to take this opportunity to thank all those people who helped me bring this thesis to completion. The Staff of Massey University History Department, in particular my supervisor Dr. Joel Hayward for first suggesting the topic, for his generosity with time and resources, and for his guidance and support in my frequent moments of uncertainty. Also a special thanks to the History Department Secretaries, Mary Lou Dickson and Lynne Coates. To Dr. Axel Laurs, my second supervisor, for patiently reading numerous drafts and always having a bottle of red wine on offer. Also to Jiirg Br6nnimann for all his assistance. Annette Holm at Massey University Library lnterloans for her unbelievable speed in acquiring books, articles, and microfilm from all over New Zealand, Germany, and America. To my family and friends, for making me hang in there and get it finished . A special thanks to the crew at George St. Deli, especially Jo, for keeping me sane and for the constant supply of caffeine. -

Germany Depth Study Content Revision Guide 1
Germany Depth Study Content Revision Guide 1. Armistice – November 1918 • Kaiser abdicated (gave up being King) 10th November • Ebert signed the German surrender (admitted defeat) 11th November • Many people, especially soldiers, were angry that they had been betrayed by the government • Ebert was known as one of the ‘November Criminals’ • The ‘Dolchtosslegende’ (‘Stab in the Back’ myth) developed as a consequence • Jews were also blamed for the Armistice – people felt they should have used their influence to help the government and the country to keep fighting 2. Spartacist Uprising, January 1919 Events: • The Spartacists were a LEFT WING Communist group who tried to overthrow Ebert • 100,000 people protested – the army REFUSED to help Ebert out because of the Armistice • Ebert called on the FREIKORPS – ex-army, had their own weapons – to sort it out • The Freikorps didn’t like Ebert, but hated communists more, so they helped • The Spartacist leaders (Liebknecht and Luxemberg) were killed • Uprising failed after a week long street fight in Berlin Effects: • Negative/Political: Showed that Ebert was weak, had no authority over his own army, and that some people did not support him • Positive/political: Ebert was able to defend his own country and after calling an election to make himself legitimate, began to establish some laws over Germany 3. Treaty of Versailles, June 1919 Terms: LAND ARMY • 13% of land lost, including… • German army reduced to 100,000 soldiers (from - Alsace-Lorraine to France 1.5M before WW1) - Saarland coalfields (50% of Germany’s coal) • No air force/No submarines • 10% of population displaced • Maximum of 6 battleships • Rhineland (border between France and Germany = demilitarised) MONEY BLAME • $6.6 billion reparations to be paid to Belgium and • ‘War Guilt Clase’ (Article 231) – Germany had to France for the damage accept total responsibility for losing WW1 Effects: • Political – made Ebert’s position difficult, Germans saw him as a traitor. -

Topic Booklet Weimar and Nazi Germany
Option 31 Topic booklet Weimar and Nazi Germany, 1918–39 GCSE (9-1) History Pearson Edexcel Level 1/Level 2 GCSE (9-1) in History (1HI0) Contents 1. Overview 2 1.1 Assessment 2 2. Content guidance 4 2.1 Summary of content 4 2.2 Content exemplification and mapping 6 3. Student timeline 16 4. Resources 18 4.1 Resources for students 18 4.2 Resources for teachers 20 © Pearson 2015 1. Overview 1. Overview In contrast to the proud, patriotic and celebratory mood with which Germany entered the First World War in 1914, it was a shocked and defeated Germany in 1918 that then bitterly resented its treatment by the victorious allies with the Treaty of Versailles. A new, democratic Weimar Germany was burdened with severe political and economic dislocation and by 1923 faced the humiliating invasion by the French of its industrial centre of the Ruhr. However, a mixture of Stresemann’s work and more favourable international conditions allowed Weimar Germany to undergo a period known as ‘the Golden Years’ until the Wall Street Crash in 1929 had significant economic and thus political consequences for Weimar Germany. Immediately at the end of the First World War, the right wing of German politics were especially resentful at Germany’s defeat, at the peace treaty and at the new democratic style of government. Hitler had become leader of the National Socialist Workers’ Party. After having attempted coming to power by means of force in Munich in November 1923, Hitler determined to achieve power by democratic means. Once power had been achieved, democratic government could then be abandoned. -

The NSDAP and the German Working Class, 1925-1933
8 The NSDAP and the German Working Class, 1925-1933 PETER D. STACHURA Throughout the pre-1933 period, the National Socialist party (NSDAP) projected an image of being a broadly-based Volksbewegung whose aim was to restore the fortunes of all Germans regardless of status or class. The party's ideological and propagandistic appeal was modelled to attract to the swastika as many sections of \~eimar society as possible. This approach made sense, after all, if the NSDAP were to exoand its electoral constituency to the point where it could establish a popular mandate for power. Following the unsuccessful 11unich putsch in 1923, Hitler renounced violent, revolutionary tactics in favor of a long-term parliamentary strategy that would allow him to assume governmental responsibility within the letter of the law. In the end, of course, the NSDAP did win power legally even if it constantly violated the spirit of the law. While failing to attain an overall majority in Reichstag elections in July and November 1932, the NSDAP, despite showing incipient signs of having passed its peak, was ultimately brought into the government, thanks to the last-minute interventionist power politics of industrial and agrarian elitist groups representing propertied, nationalist, and Protestant Germany.l Contrary to Joseph Goebbels's assertion in early 1933 that the !1achtergreifung signified "a revolution of a workers' movement,"2 empirical historical inquiry has established that by 1933 the NSDAP drew its electoral support overwhelmingly from the small town and rural Protestant Mittelstand, comprising men and women in roughly equal numbers, in northern, central, and eastern Germany.3 Although by 1930-31 the lower 11ittelstand, particularly of the "old" or traditional type, predominated among the party's voters and members, the upper Hittelstand were beginning to flock into the ranks in ever-increasing numbers in 1932,4 thus making the NSDAP more of a catch-all movement of middle-class protest, a movement of bourgeois integration. -

Hitler's Rise to Power, 1919-33
HISTORY – YEAR 11 – HITLER’S RISE TO POWER, 1919- 33 A KEY DATES C KEY TERMS/CONCEPTS 1 Sept 1919 Hitler joins German Workers Party. German Workers Party – later the NSDAP or ‘Nazi’ Party. A 1 DAP right-wing, nationalist political organisation. 2 24th Feb 1920 Twenty-Five Point programme. Twenty-Five Point A set of 25 beliefs and policies that the Nazis would introduce 3 Oct 1921 SA formed. 2 Programme if elected. It included anti-Semitic points. 4 Nov 1923 Munich Putsch – Hitler arrested. Hitler’s private army of stormtroopers, known as the 3 SA 5 1924 Hitler writes ‘Mein Kampf’ in prison. ‘brownshirts’. 6 Feb 1926 Bamberg conference – Führerprinzip. Hitler’s failed attempt to seize power in Munich. It gave the 4 Munich Putsch Nazis a great deal of publicity. 7 Late 1929 Wall Street Crash. Meaning ‘My Struggle’ – book written by Hitler in prison 14th Sept 5 Mein Kampf 8 Nazis win 107 seats in September elections. outlining his vision for making Germany strong internationally. 1930 Bamberg Held to establish the idea of Fuhrerprinzip – the Fuhrer as 31st July 6 9 Nazis win 230 seats as unemployment rises Conference sole leader. 1932 Reached over 6 million in 1933. Many turned to extreme 10 30th Jan 1933 Hitler becomes Chancellor of Germany. 7 Unemployment B KEY PEOPLE parties. 1 Anton Drexler Founded the Nazi Party and co-wrote the 25 points. Many workers supported the Communist Party. Businesses 8 Communist Party supported the Nazis as a result and funded Hitler’s campaign. 2 Adolf Hitler Leader of NSDAP (Nazi Party). -

Nazi Party 3
Student name: __________________________________________ Timeline Key Events Class code/teacher: ______________________________________ 1 1919 Hitler joins German 15 Hitler’s early 1. Devastated by the death of his mother in 1907 _______________________________________________________ Workers Party (DAP) Life 2. Shortly after he was rejected from art college, destroying his dream of becoming an artist 3. For the next 5 years Hitler slept rough in parks and earned pennies painting postcards 2 1920 25 Point Programme released – DAP changes 16 Hitler in 1. Hitler volunteered to fight in World War I it’s name to National World War I 2. He won the highest German medal for bravery. The Iron Cross First Class Socialist German Workers 3. He felt betrayed by the Weimar Politicians signed the armistice. He saw them as ‘November Criminals’ Party (NSDAP) or Nazi for 4. He believed in the ‘Doltchstoss’ – ‘stab in the back’ myth. That communists and Jews had betrayed Germany by causing the armistice. short. 17 Early Nazi 1. 1919 Hitler joins the DAP. Hitler started making speeches at meetings and discovered he was good at public speaking 3 1921 Hitler becomes leader of Party 2. He discovered that people agreed with the topics he spoke about e.g. November Criminals, Dolchtoss, hatred of the ToV, hatred of Jews, Hatred of communists. the Nazi Party 3. 1920 Nazis publish 25 Point Plan. Included ideas about cratering an Empire, excluding Jews from society and destroying the ToV 4. 1921 becomes leader- ‘Fuhrer’ - of Nazi Party. Hitler decides that he should have ultimate power and be questioned by no one. This called the Fuhrerprinzip 4 Nov. -

The Weimar Republic 1918-1919
Weimar and Nazi Germany. 1918-1939 Personal Learning Checklist Below is a list of the content you will need to know for the Weimar and Nazi Germany unit You will need to about ALL of the topics. 1. The legacy of WW1. The abdication of the Kaiser, the armistice and revolution, 1918-19. The Origins of the Republic, The setting up if the Weimar Republic. The 1918-19 strengths and weaknesses of the new constitution. 2. Reasons for the early unpopularity of the Republic, including the ‘stab in the back’ theory and the key The early terms of the Treaty of Versailles. challenges to The Weimar the Weimar Challenges to the Republic from Left and Right. Republic Republic, 1919- Spartacists, Freikorps, the Kapp Putsch. 23 1918-1919 The challenges of 1923, hyperinflation; reasons for, and effect of, the French occupation of the Ruhr. 3. Reasons for economic recovery, including the work of Stresemann, the Rentenmark the Dawes and The recovery Young Plans and American loans/investment of the Republic 1924-29 Impact of domestic policies of Stresemann’s activities abroad: Locarno Pact, joining the League of Nations and the Kellogg-Briand Pact. 4. Changes in the stand of living, including wages, housing and unemployment insurance Changes in society, 1924- Changes in the position of women in work, politics 1929 and leisure Cultural changes: developments in architecture, art and cinema. 1. Hitler’s early career: joining the German Workers’ Party and setting up the Nazi Party, 1919-20. Early development of the Nazi The early growth and features of the Party. -
Germany Knowledge Organisers PDF File
How do we revise with our Knowledge Organisers? Teach it! Flash Cards Record It Teach someone your key Write the key word or date on Record yourself on facts and the get them one side and the explanation your phone or tablet to test you, or even test on the other. Test your reading out the them! memory by asking someone to information. These quiz you on either side. can be listened to as many times as you Hide and Seek want! Read through your Back to front knowledge organiser, put it down and try and write Write down the answers out as much as you can and then write out what remember. Then keep the questions the adding to it until its full! Sketch it teacher may ask to get those answers. Draw pictures to Read Aloud represent each of the facts or Simply speak the dates. It could facts and dates be a simple out loud as you’re drawing or reading the something that Knowledge Organiser. reminds you of Practice! Even try to act out the answer. some of the facts – it Some find they remember really helps you by simply writing the facts remember! over and over again. Germany Knowledge Organiser – Weimar Germany 1919-‐29 Timeline Key Words 1. 9th Nov 1918 Kaiser abdicated (leaves the throne) and flees 17. Armistice Agreement to stop fighting, Germany asked for it in 1918 Germany 18. November Weimar politicians blamed for the ‘Stab in the Back’ of 2. 9th Nov 1918 The Weimar Republic is set up. Criminals Germany by surrendering at the end of World War One.