Nitrates and Salinity CD No Maps
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2011 Population and Housing Census
2011 POPULATION AND HOUSING CENSUS Ministry of Finance and Development Planning 2011 Census Slogan: Palo yame, tsela ya ditlhabololo My count, a guide to developments August 2009 CSO in Collaboration with UNFPA PROJECT DOCUMENT 2011 POPULATION AND HOUSING CENSUS Published by Central Statistics Office Private Bag 0024, Gaborone Telephone: 33671300 Fax: 3952201 E-mail:[email protected] Website: www.cso.gov.bw Contact Unit : Census Secretariat Telephone: 3671300 Ext. 1305 August 2009 COPYRIGHT RESERVED Extracts may be published if source is duly acknowledged i CONTENTS PREFACE .................................................................................................................... 1 LIST OF ABBREVIATIONS AND ACRONYMS ....................................................... 2 EXECUTIVE SUMMARY ........................................................................................... 4 1. BACKGROUND AND JUSTIFICATION ................................................................ 5 1.1 Background of the 2011 Census Taking .............................................................. 5 1.2 Justification ......................................................................................................... 5 1.2.1 Evidence-based decision making, policy-making, planning and administration ....................................................................................................... 5 1.2.2 Research .................................................................................................... 5 1.2.3 Service to stakeholders -

2017 SEAT Report Orapa, Letlhakane and Damtshaa Mines
SEAT 3 REPORT Debswana - Orapa, Letlhakane and Damtshaa Mine (OLDM) November 2017 1 2 FOREWORD rounded assessment of the socio-economic impacts of our operation – positive and negative - in our zone of influence. This is the second impact assessment of this nature being conducted, the first one having been carried out in 2014. The information in this socio-economic assessment report helped to improve our understanding of the local dynamics associated with the impact our operation’s that are real and perceived. It also provided us with invaluable insight into our stakeholders’ perspectives, expectations, concerns and suggestions. This report is a valuable tool to guide our thinking on community development and our management of the social impacts of OLDM’s future closure at the end of the life of mine. Furthermore, it provides a useful mechanism in mobilising local stakeholders to work with us towards successful mine closure. The report has also given valuable feedback on issues around mine expansion initiatives and access to resources like land and groundwater. More importantly, meaningful feedback has been provided around two critical areas: resettlement and options around town transformation. We recognise the complexity of a proper plan for future mine closure at the end of the life of mine. In the past, our plans for future mine closure mainly focused on environmental aspects, with community involvement often limited to cursory consultation processes. Today, in line with current trends, my team and I are convinced that community ownership of the post closure goals is the only sustainable means to propel communities to prosper when OLDM is no longer involved. -

Bank of Botswana
PAPER 4 BANK OF BOTSWANA DIRECTORY OF FINANCIAL INSTITUTIONS OPERATING IN BOTSWANA AS AT DECEMBER 31, 2009 PREPARED AND DISTRIBUTED BY THE BANKING SUPERVISION DEPARTMENT BANK OF BOTSWANA Foreword This directory is compiled and distributed by the Banking Supervision Department of the Bank of Botswana. While every effort has been made to ensure the accuracy of the information contained in this directory, such information is subject to frequent revision, and thus the Bank accepts no responsibility for the continuing accuracy of the information. Interested parties are advised to contact the respective financial institutions directly for any information they require. This directory excludes Collective Investment Undertakings and International Financial Services Centre non-bank entities, whose regulation and supervision have been transferred to the Non-Bank Financial Institutions Regulatory Authority. Oabile Mabusa DIRECTOR BANKING SUPERVISION DEPARTMENT 1 DIRECTORY OF FINANCIAL INSTITUTIONS OPERATING IN BOTSWANA TABLE OF CONTENTS 1. CENTRAL BANK ............................................................................................................................................. 4 2. COMMERCIAL BANKS ................................................................................................................................... 6 2.1 ABN AMRO BANK (B OTSWANA ) LIMITED ..................................................................................................... 6 2.2 ABN AMRO BANK (B OTSWANA ) OBU LIMITED ........................................................................................... -

Botswana Semiology Research Centre Project Seismic Stations In
BOTSWANA SEISMOLOGICAL NETWORK ( BSN) STATIONS 19°0'0"E 20°0'0"E 21°0'0"E 22°0'0"E 23°0'0"E 24°0'0"E 25°0'0"E 26°0'0"E 27°0'0"E 28°0'0"E 29°0'0"E 30°0'0"E 1 S 7 " ° 0 0 ' ' 0 0 ° " 7 S 1 KSANE Kasane ! !Kazungula Kasane Forest ReserveLeshomo 1 S Ngoma Bridge ! 8 " ! ° 0 0 ' # !Mabele * . MasuzweSatau ! ! ' 0 ! ! Litaba 0 ° Liamb!ezi Xamshiko Musukub!ili Ivuvwe " 8 ! ! ! !Seriba Kasane Forest Reserve Extension S 1 !Shishikola Siabisso ! ! Ka!taba Safari Camp ! Kachikau ! ! ! ! ! ! Chobe Forest Reserve ! !! ! Karee ! ! ! ! ! Safari Camp Dibejam!a ! ! !! ! ! ! ! X!!AUD! M Kazuma Forest Reserve ! ShongoshongoDugamchaRwelyeHau!xa Marunga Xhauga Safari Camp ! !SLIND Chobe National Park ! Kudixama Diniva Xumoxu Xanekwa Savute ! Mah!orameno! ! ! ! Safari Camp ! Maikaelelo Foreset Reserve Do!betsha ! ! Dibebe Tjiponga Ncamaser!e Hamandozi ! Quecha ! Duma BTLPN ! #Kwiima XanekobaSepupa Khw!a CHOBE DISTRICT *! !! ! Manga !! Mampi ! ! ! Kangara # ! * Gunitsuga!Njova Wazemi ! ! G!unitsuga ! Wazemi !Seronga! !Kaborothoa ! 1 S Sibuyu Forest Reserve 9 " Njou # ° 0 * ! 0 ' !Nxaunxau Esha 12 ' 0 Zara ! ! 0 ° ! ! ! " 9 ! S 1 ! Mababe Quru!be ! ! Esha 1GMARE Xorotsaa ! Gumare ! ! Thale CheracherahaQNGWA ! ! GcangwaKaruwe Danega ! ! Gqose ! DobeQabi *# ! ! ! ! Bate !Mahito Qubi !Mahopa ! Nokaneng # ! Mochabana Shukumukwa * ! ! Nxabe NGAMILAND DISTRICT Sorob!e ! XurueeHabu Sakapane Nxai National Nark !! ! Sepako Caecae 2 ! ! S 0 " Konde Ncwima ° 0 ! MAUN 0 ' ! ! ' 0 Ntabi Tshokatshaa ! 0 ° ! " 0 PHDHD Maposa Mmanxotai S Kaore ! ! Maitengwe 2 ! Tsau Segoro -

The Magnitude of Termites to the Future of Kimberlite Exploration in Botswana
11th International Kimberlite Conference Extended Abstract No. 11IKC-4555, 2017 The magnitude of termites to the future of kimberlite exploration in Botswana Leon R.M. Daniels, Tshireletso A. Dira and Onesimo Kufandikamwe Pangolin Diamonds (Pty) Limited, Tatitown, Botswana [email protected], [email protected], [email protected] Introduction The majority of the diamond mines in Botswana (Orapa, Jwaneng, Letlhakane, Damsthaa, Lerala and BK11) were discovered as a direct consequence of soil sampling for indicator minerals such as garnet and picroilmenite. Over the past sixty years the application of soil sampling for indicator minerals as a primary exploration tool has diminished while aeromagnetic surveys have increased in popularity. The rate of kimberlite discovery in Botswana over the past fifteen years has declined significantly. The obvious magnetic kimberlites have been discovered. The future of new kimberlite discoveries, mainly poorly magnetic to non-magnetic, is once again dependent on soil sampling for kimberlite indicator minerals. The only mechanism to transport indicators from source to surface through the Kalahari Formation overburden is through termite bioturbation. Soil Sampling History in Botswana Initial soil sampling programmes conducted by De Beers and Central African Selection Trust (CAST) in Botswana (then Bechuanaland) during the mid to late 1950’s were confined to stream sediments in areas where drainage was developed. Concentrates were generated using gold pans. This method was quite ineffective and possibly resulted in Jwaneng being missed in a soil sampling programme conducted in 1962 (Lamont, 2011). A “semi-continuous scoop” sampling method was developed to sample interfluves areas and eventually into the Kalahari sandveld. The gold pan was abandoned as a concentrating tool and a gravitating screen, commonly used by diamond diggers, was introduced to concentrate soil samples between 2mm and 0.5mm in size. -
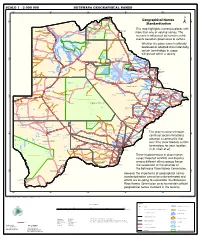
Geographical Names Standardization BOTSWANA GEOGRAPHICAL
SCALE 1 : 2 000 000 BOTSWANA GEOGRAPHICAL NAMES 20°0'0"E 22°0'0"E 24°0'0"E 26°0'0"E 28°0'0"E Kasane e ! ob Ch S Ngoma Bridge S " ! " 0 0 ' ' 0 0 ° Geographical Names ° ! 8 !( 8 1 ! 1 Parakarungu/ Kavimba ti Mbalakalungu ! ± n !( a Kakulwane Pan y K n Ga-Sekao/Kachikaubwe/Kachikabwe Standardization w e a L i/ n d d n o a y ba ! in m Shakawe Ngarange L ! zu ! !(Ghoha/Gcoha Gate we !(! Ng Samochema/Samochima Mpandamatenga/ This map highlights numerous places with Savute/Savuti Chobe National Park !(! Pandamatenga O Gudigwa te ! ! k Savu !( !( a ! v Nxamasere/Ncamasere a n a CHOBE DISTRICT more than one or varying names. The g Zweizwe Pan o an uiq !(! ag ! Sepupa/Sepopa Seronga M ! Savute Marsh Tsodilo !(! Gonutsuga/Gonitsuga scenario is influenced by human-centric Xau dum Nxauxau/Nxaunxau !(! ! Etsha 13 Jao! events based on governance or culture. achira Moan i e a h hw a k K g o n B Cakanaca/Xakanaka Mababe Ta ! u o N r o Moremi Wildlife Reserve Whether the place name is officially X a u ! G Gumare o d o l u OKAVANGO DELTA m m o e ! ti g Sankuyo o bestowed or adopted circumstantially, Qangwa g ! o !(! M Xaxaba/Cacaba B certain terminology in usage Nokaneng ! o r o Nxai National ! e Park n Shorobe a e k n will prevail within a society a Xaxa/Caecae/Xaixai m l e ! C u a n !( a d m a e a a b S c b K h i S " a " e a u T z 0 d ih n D 0 ' u ' m w NGAMILAND DISTRICT y ! Nxai Pan 0 m Tsokotshaa/Tsokatshaa 0 Gcwihabadu C T e Maun ° r ° h e ! 0 0 Ghwihaba/ ! a !( o 2 !( i ata Mmanxotae/Manxotae 2 g Botet N ! Gcwihaba e !( ! Nxharaga/Nxaraga !(! Maitengwe -

BNSC Annual Report 2018
1 Board & Department Affiliates Financial Management Reports Reports Report 2 CONTENTS BNSC Vision 2028 BOARD & MANAGEMENT 2 - 11 The Vision statement captures the “desired future state” Board Members 2 - 3 of the Statement By The Chairman 4 - 5 Organisation - what the BNSC aspires Chief Executive Officer’s Report 6 - 9 to be in the future. Management 10 - 11 The basis for the 16 year Strategy Horizon is as follows; DEPARTMENTAL REPORTS 14 - 23 Games Department 12 - 17 The period aligns to the 4 year Olympic Games Human Resources And Administration Department 18 - 20 cycle. Sport Development Department Report 19 - 20 Internal Audit Department 21 The period takes into consideration the time Lands & Facilities Department 22 - 23 required to develop athletes from the grass Business Development Department 24 - 25 roots level at an appropriate young age (6 year Sport Development Department 26 - 28 old) to elite and professional levels. NATIONAL SPORT ASSOCIATION 30 - 91 The BNSC vision statement embodies the following strategic aspirations; FINANCIAL REPORT 92 - 134 • Sport for ALL- All Batswana actively participating in sports and/or physical activity • Sport for Excellence- Professional and elite athletes achieving sustained superior performance on the world stage. • Sport for Prosperity- Sport as a development partner contributing significantly to economic diversification. Botswana hosting prestigious sporting events that contribute to national pride and contribute significant socio-economic benefits. 3 Board & Department Affiliates Financial Management Reports Reports Report Board Members 1 2 3 4 5 6 1. Solly Reikeletseng 4. Gift Nkwe Chairman Member 2. Prof. Martin Mokgwathi 5. Kago Ramokate Member Member 3. Shirley Keoagile 6. -

Botswana Population Projections 2011-2026
STATISTICS BOTSWANA BOTSWANA POPULATION PROJECTIONS 2011 – 2026 Statistics Botswana. Private Bag 0024 Botswana Tel: (267) 367 1300. Fax: (267) 395 2201. Email: [email protected] Website: www.cso.gov.bw POPULATION PROJECTIONS FOR BOTSWANA 2011-2026 Published by STATISTICS BOTSWANA Private Bag 0024, Gaborone Phone: (267)3671300, Fax: (267) 3952201 Email: [email protected] Website: www.cso.gov.bw Contact: Census and Demographic Analysis Unit Census and Demography Division Printed and obtainable from STATISTICS BOTSWANA Private Bag 0024, Gaborone Phone: (267)3671300, Fax: (267) 3952201 November 2015 COPYRIGHT RESERVED PREFACE This Population Projections Report is another product of a series of reports that are derived from the 2011 Population and Housing Census data. It is one of the main volumes in the 2011 Population and Housing Census series of publications. The report contains the projected counts of the future population. Unlike in the previous projections, which projected for a 30 year period, the current report projects the population for the next 15 years using the 2011 census data as the base population. These population projections are presented at national, district. A report on locality population projections will be published separately. The main purpose of producing population projections is to provide an estimate of the future population as a common framework for use in planning, policy formulation and decision making in various sectors of the economy. This would assist the planning process in presenting a future outlook of the population. This includes among others; informing the allocation of the resources from the central government to local areas and plan ahead for the provision of servies. -

Copyright Government of Botswana CHAPTER 69:04
CHAPTER 69:04 - PUBLIC ROADS: SUBSIDIARY LEGISLATION INDEX TO SUBSIDIARY LEGISLATION Declaration of Public Roads and Width of Public Roads Order DECLARATION OF PUBLIC ROADS AND WIDTH OF PUBLIC ROADS ORDER (under section 2 ) (11th March, 1960 ) ARRANGEMENT OF PARAGRAPHS PARAGRAPHS 1. Citation 2. Establishment and declaration of public roads 3. Width of road Schedule G.N. 5, 1960, L.N. 84, 1966, G.N. 46, 1971, S.I. 106, 1971, S.I. 94, 1975, S.I. 95, 1975, S.I. 96, 1975, S.I. 97, 1982, S.I. 98, 1982, S.I. 99, 1982, S.I. 100, 1982, S.I. 53, 1983, S.I. 90, 1983, S.I. 6, 1984, S.I. 7, 1984, S.I. 151, 1985, S.I. 152, 1985. 1. Citation This Order may be cited as the Declaration of Public Roads and Width of Public Roads Order. 2. Establishment and declaration of public roads The roads described in the Schedule hereto are established and declared as public roads. 3. Width of road The width of every road described in the Schedule hereto shall be 30,5 metres on either side of the general run of the road. SCHEDULE Description District Distance in kilometres RAMATLABAMA-LOBATSE Southern South 48,9 East Commencing at the Botswana-South Africa border at Ramatlabama and ending at the southern boundary of Lobatse Township as shown on Plan BP225 deposited with the Director of Surveys and Lands, Gaborone. LOBATSE-GABORONE South East 65,50 Copyright Government of Botswana ("MAIN ROAD") Leaving the statutory township boundary of Lobatse on the western side of the railway and entering the remainder of the farm Knockduff No. -

Experience of Asphalt Botswana (Pty) Ltd in Works of Civil Engineering Nature
EXPERIENCE OF ASPHALT BOTSWANA (PTY) LTD IN WORKS OF CIVIL ENGINEERING NATURE PROJECT NAME AND LOCATION EMPLOYER DESCRIPTION OF WORK PROJECT VALUE YEAR OF COMPLETION 1. Construction of Gaborone/Boatle road Ministry of Transport and Asphalt Surfacing works P64 Million 2019 to Dual Carriageway Communications Tel: 3913511 CCC/CSCEC JV Tel: +267 316 5373 2. Mankgodi - Kanye Junction – Jwaneng Ministry of Transport and Asphalt Surfacing works and Double seal road Rehabilitation Communications Tel: surface treatment 3913511 Contract: P130 Mil 2019 Elsamex International Tel: +267 544-1151 3. Dibete-Mookane-Machaneng Road Ministry of Transport and Road Construction, Drainage & Double (132km) Communications Tel: Seal Surface Treatment 3913511 Overall: P524 Mil December 2019 Joint Venture with EH Botswana (Pty) Ltd 4. Gabane Village Infrastructure Ministry of Lands and Construction of roads, drainage structures, Development Housing sewerage and portable water Overall: P214 Mil November 2019 Tel: 3682000 5. Orapa Airport Refurbishment Phase 2 Debswana Diamond Civil, Structural and Electrical works Contract: P15 Mil December 2017 Company, Tel: 2904008 6. Phakalane Glen Valley Road Ministry of Lands and Asphalt Surfacing works Gaborone, Botswana Housing Tel: 3682000 Contract: P8.2mil 2016 Landmark Projects Tel: 3933678 7. Tshele Hills Road Over Rail Ministry of Transport and Asphalt Surfacing for road over rail project Rasesa, Botswana Communications Tel: on A1 road at Rasesa 3913511 Contract: P14.8mil 2016 Unik Construction Tel: 3924965 8. Letlhakeng Infrastructure Kweneng District Council Double seal surface treatment Letlhakeng, Botswana Tel: 5920200 Contract: P11.2mil 2016 Precon Construction Page 1 of 2 EXPERIENCE OF ASPHALT BOTSWANA (PTY) LTD IN WORKS OF CIVIL ENGINEERING NATURE Tel: 3956629 9. -

Republic of Botswana the Project for Enhancing National Forest Monitoring System for the Promotion of Sustainable Natural Resource Management
DEPARTMENT OF FORESTRY AND RANGE RESOURCES (DFRR) MINISTRY OF ENVIRONMENT, NATURAL RESOURCES CONSERVATION AND TOURISM (MENT) REPUBLIC OF BOTSWANA REPUBLIC OF BOTSWANA THE PROJECT FOR ENHANCING NATIONAL FOREST MONITORING SYSTEM FOR THE PROMOTION OF SUSTAINABLE NATURAL RESOURCE MANAGEMENT PROJECT COMPLETION REPORT DECEMBER 2017 JAPAN INTERNATIONAL COOPERATION AGENCY(JICA) ORIENTAL CONSULTANTS GLOBAL CO., LTD. JAPAN FOREST TECHNOLOGY ASSOCIATION GE JR 17-131 DEPARTMENT OF FORESTRY AND RANGE RESOURCES (DFRR) MINISTRY OF ENVIRONMENT, NATURAL RESOURCES CONSERVATION AND TOURISM (MENT) REPUBLIC OF BOTSWANA REPUBLIC OF BOTSWANA THE PROJECT FOR ENHANCING NATIONAL FOREST MONITORING SYSTEM FOR THE PROMOTION OF SUSTAINABLE NATURAL RESOURCE MANAGEMENT PROJECT COMPLETION REPORT DECEMBER 2017 JAPAN INTERNATIONAL COOPERATION AGENCY(JICA) ORIENTAL CONSULTANTS GLOBAL CO., LTD. JAPAN FOREST TECHNOLOGY ASSOCIATION DFRR/JICA: Botswana Forest Distribution Map Zambia Angola Zambia Legend KASANE Angola ! ! Settlement CountryBoundary Riparian Forest Typical Forest Woodland Zimbabwe Zimbabwe Bushland/Shrubland Savanna/Grassland/Forbs MAUN ! NATA Baregorund ! TUTUME ! Desert/Sand Dunes Marsh/Wetland FRANCISTOWN Waterbody/Pan ! ORAPA Namibia ! TONOTA ! GHANZI Angola Zambia Namibia ! SELEBI-PHIKWE BOBONONG ! ! Zimbabwe SEROWE ! PALAPYE ! Namibia MAHALAPYE ! South Africa KANG ! MOLEPOLOLE MOCHUDI ! ! JWANENG ! GABORONE ! ´ 0 50 100 200 RAMOTSWA ! KANYE Kilometres ! Coordinate System: GCS WGS 1984 Datum: WGS 1984 LOBATSE ! Botswana Forest Distribution Map Produced from -

Orapa Business Park Location Analysis
ORAPA BUSINESS PARK LOCATION ANALYSIS PRESENTATION TO THE ORAPA,LETLHAKANE AND DAMTSHAA MINES (OLDM) COMMITEE 16 TH AUGUST 2013 STRUCTURE OF PRESENTATION . Introduction . Project Area . Location Analysis o Regional Scale o Local Scale o Site Alternatives . Implementation Plan INTRODUCTION Background Information Project Purpose BACKGROUND INFORMATION • Business Park Sub Committee has been tasked with identifying the best geographic location for an industrial/business park to support mining activities in the Orapa/Letlhakane Area. • The area around the OLD Mines has been identified as a potential location for the industrial park due to its strategic location. • The activities within the industrial park are envisaged to include Processing; Machinery; Workshops; Skills Training; Logistics; Offices & hotel; and Trade • The discussion below is a preliminary analysis regarding the above PROJECT AREA Location Settlement Size Population PROJECT AREA • The project area is situated in Central District; within Boteti Sub District. • Major settlements in the Project Area are Letlhakane Village and ORAPA LETLHAKANE Orapa Mining Town. Location Analysis (Regional) Strategic Linkages Regional Context Strategic Linkages Well networked through regional linkages; MAUN • Orapa-F/Town • Orapa- Serowe FRANCISTOWN • Francistown-Maun ORAPA LETLHAKANE • F/town - Gaborone SEROWE GABORONE AIRPORT ORAPA MINE DAMTSHAA ORAPA LETLHAKANE LETLHAKANE MINE Location Analysis (Local) Assessing Dominant Location Factors Alternative Sites Evaluating Alternaives