MCRS1 Associates with Cytoplasmic Dynein and Mediates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4-6 Weeks Old Female C57BL/6 Mice Obtained from Jackson Labs Were Used for Cell Isolation
Methods Mice: 4-6 weeks old female C57BL/6 mice obtained from Jackson labs were used for cell isolation. Female Foxp3-IRES-GFP reporter mice (1), backcrossed to B6/C57 background for 10 generations, were used for the isolation of naïve CD4 and naïve CD8 cells for the RNAseq experiments. The mice were housed in pathogen-free animal facility in the La Jolla Institute for Allergy and Immunology and were used according to protocols approved by the Institutional Animal Care and use Committee. Preparation of cells: Subsets of thymocytes were isolated by cell sorting as previously described (2), after cell surface staining using CD4 (GK1.5), CD8 (53-6.7), CD3ε (145- 2C11), CD24 (M1/69) (all from Biolegend). DP cells: CD4+CD8 int/hi; CD4 SP cells: CD4CD3 hi, CD24 int/lo; CD8 SP cells: CD8 int/hi CD4 CD3 hi, CD24 int/lo (Fig S2). Peripheral subsets were isolated after pooling spleen and lymph nodes. T cells were enriched by negative isolation using Dynabeads (Dynabeads untouched mouse T cells, 11413D, Invitrogen). After surface staining for CD4 (GK1.5), CD8 (53-6.7), CD62L (MEL-14), CD25 (PC61) and CD44 (IM7), naïve CD4+CD62L hiCD25-CD44lo and naïve CD8+CD62L hiCD25-CD44lo were obtained by sorting (BD FACS Aria). Additionally, for the RNAseq experiments, CD4 and CD8 naïve cells were isolated by sorting T cells from the Foxp3- IRES-GFP mice: CD4+CD62LhiCD25–CD44lo GFP(FOXP3)– and CD8+CD62LhiCD25– CD44lo GFP(FOXP3)– (antibodies were from Biolegend). In some cases, naïve CD4 cells were cultured in vitro under Th1 or Th2 polarizing conditions (3, 4). -

Pinx1: Structure, Regulation and Its Functions in Cancer
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 7, No. 40 Review PinX1: structure, regulation and its functions in cancer Hai-Long Li1,2,*, Jun Song3,4,*, Hong-Mei Yong5,*, Ping-Fu Hou1,3, Yan-Su Chen1,3, Wen-Bo Song1, Jin Bai1 and Jun-Nian Zheng1,3 1 Jiangsu Key Laboratory of Biological Cancer Therapy, Xuzhou Medical College, Xuzhou, Jiangsu, China 2 Department of Urology, The Affiliated Hospital of Xuzhou Medical College, Xuzhou, Jiangsu, China 3 Jiangsu Center for the Collaboration and Innovation of Cancer Biotherapy, Cancer Institute, Xuzhou Medical College, Xuzhou, Jiangsu, China 4 Department of General Surgery, The Affiliated Hospital of Xuzhou Medical College, Xuzhou, Jiangsu, China 5 Department of Medical Oncology, Huai’an Hospital to Xuzhou Medical College, Huai’an, Jiangsu, China * These authors have contributed equally to this study Correspondence to: Jun-Nian Zheng, email: [email protected] Correspondence to: Jin Bai, email: [email protected] Keywords: PinX1; cancer; structure; regulation; function Received: March 14, 2016 Accepted: August 09, 2016 Published: August 19, 2016 ABSTRACT PIN2/TRF1-interacting telomerase inhibitor 1 (PinX1) is a novel cloned gene located at human chromosome 8p23, playing a vital role in maintaining telomeres length and chromosome stability. It has been demonstrated to be involved in tumor genesis and progression in most malignancies. However, some researches showed opposing molecular status of PinX1 gene and its expression patterns in several other types of tumors. The pathogenic mechanism of PinX1 expression in human malignancy is not yet clear. Moreover, emerging evidence suggest that PinX1 (especially its TID domain) might be a potential new target cancer treatment. -
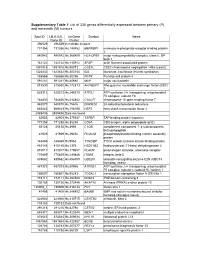
Supplementary Table 1 List of 335 Genes Differentially Expressed Between Primary (P) and Metastatic (M) Tumours
Supplementary Table 1 List of 335 genes differentially expressed between primary (P) and metastatic (M) tumours Spot ID I.M.A.G.E. UniGene Symbol Name Clone ID Cluster 296529 296529 In multiple clusters 731356 731356 Hs.140452 M6PRBP1 mannose-6-phosphate receptor binding protein 1 840942 840942 Hs.368409 HLA-DPB1 major histocompatibility complex, class II, DP beta 1 142122 142122 Hs.115912 AFAP actin filament associated protein 1891918 1891918 Hs.90073 CSE1L CSE1 chromosome segregation 1-like (yeast) 1323432 1323432 Hs.303154 IDS iduronate 2-sulfatase (Hunter syndrome) 788566 788566 Hs.80296 PCP4 Purkinje cell protein 4 591281 591281 Hs.80680 MVP major vault protein 815530 815530 Hs.172813 ARHGEF7 Rho guanine nucleotide exchange factor (GEF) 7 825312 825312 Hs.246310 ATP5J ATP synthase, H+ transporting, mitochondrial F0 complex, subunit F6 784830 784830 Hs.412842 C10orf7 chromosome 10 open reading frame 7 840878 840878 Hs.75616 DHCR24 24-dehydrocholesterol reductase 669443 669443 Hs.158195 HSF2 heat shock transcription factor 2 2485436 2485436 Data not found 82903 82903 Hs.370937 TAPBP TAP binding protein (tapasin) 771258 771258 Hs.85258 CD8A CD8 antigen, alpha polypeptide (p32) 85128 85128 Hs.8986 C1QB complement component 1, q subcomponent, beta polypeptide 41929 41929 Hs.39252 PICALM phosphatidylinositol binding clathrin assembly protein 148469 148469 Hs.9963 TYROBP TYRO protein tyrosine kinase binding protein 415145 415145 Hs.1376 HSD11B2 hydroxysteroid (11-beta) dehydrogenase 2 810017 810017 Hs.179657 PLAUR plasminogen activator, -

Variation in Protein Coding Genes Identifies Information
bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 1 1 2 3 4 5 Variation in protein coding genes identifies information flow as a contributor to 6 animal complexity 7 8 Jack Dean, Daniela Lopes Cardoso and Colin Sharpe* 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Institute of Biological and Biomedical Sciences 25 School of Biological Science 26 University of Portsmouth, 27 Portsmouth, UK 28 PO16 7YH 29 30 * Author for correspondence 31 [email protected] 32 33 Orcid numbers: 34 DLC: 0000-0003-2683-1745 35 CS: 0000-0002-5022-0840 36 37 38 39 40 41 42 43 44 45 46 47 48 49 Abstract bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 2 1 Across the metazoans there is a trend towards greater organismal complexity. How 2 complexity is generated, however, is uncertain. Since C.elegans and humans have 3 approximately the same number of genes, the explanation will depend on how genes are 4 used, rather than their absolute number. -

Supplementary Materials
Supplementary materials Supplementary Table S1: MGNC compound library Ingredien Molecule Caco- Mol ID MW AlogP OB (%) BBB DL FASA- HL t Name Name 2 shengdi MOL012254 campesterol 400.8 7.63 37.58 1.34 0.98 0.7 0.21 20.2 shengdi MOL000519 coniferin 314.4 3.16 31.11 0.42 -0.2 0.3 0.27 74.6 beta- shengdi MOL000359 414.8 8.08 36.91 1.32 0.99 0.8 0.23 20.2 sitosterol pachymic shengdi MOL000289 528.9 6.54 33.63 0.1 -0.6 0.8 0 9.27 acid Poricoic acid shengdi MOL000291 484.7 5.64 30.52 -0.08 -0.9 0.8 0 8.67 B Chrysanthem shengdi MOL004492 585 8.24 38.72 0.51 -1 0.6 0.3 17.5 axanthin 20- shengdi MOL011455 Hexadecano 418.6 1.91 32.7 -0.24 -0.4 0.7 0.29 104 ylingenol huanglian MOL001454 berberine 336.4 3.45 36.86 1.24 0.57 0.8 0.19 6.57 huanglian MOL013352 Obacunone 454.6 2.68 43.29 0.01 -0.4 0.8 0.31 -13 huanglian MOL002894 berberrubine 322.4 3.2 35.74 1.07 0.17 0.7 0.24 6.46 huanglian MOL002897 epiberberine 336.4 3.45 43.09 1.17 0.4 0.8 0.19 6.1 huanglian MOL002903 (R)-Canadine 339.4 3.4 55.37 1.04 0.57 0.8 0.2 6.41 huanglian MOL002904 Berlambine 351.4 2.49 36.68 0.97 0.17 0.8 0.28 7.33 Corchorosid huanglian MOL002907 404.6 1.34 105 -0.91 -1.3 0.8 0.29 6.68 e A_qt Magnogrand huanglian MOL000622 266.4 1.18 63.71 0.02 -0.2 0.2 0.3 3.17 iolide huanglian MOL000762 Palmidin A 510.5 4.52 35.36 -0.38 -1.5 0.7 0.39 33.2 huanglian MOL000785 palmatine 352.4 3.65 64.6 1.33 0.37 0.7 0.13 2.25 huanglian MOL000098 quercetin 302.3 1.5 46.43 0.05 -0.8 0.3 0.38 14.4 huanglian MOL001458 coptisine 320.3 3.25 30.67 1.21 0.32 0.9 0.26 9.33 huanglian MOL002668 Worenine -

Expression of MCRS1 and MCRS2 and Their Correlation with Serum Carcinoembryonic Antigen in Colorectal Cancer
EXPERIMENTAL AND THERAPEUTIC MEDICINE 12: 589-596, 2016 Expression of MCRS1 and MCRS2 and their correlation with serum carcinoembryonic antigen in colorectal cancer CHENGUANG LI1,2, MINGXIAO CHEN1, PINGWEI ZHAO1, DESALEGN ADMASSU AYANA3, LEI WANG1 and YANFANG JIANG2 1Department of Colorectal and Anal Surgery, The First Hospital, Jilin University, Changchun, Jilin 130032; 2Key Laboratory of Zoonosis Research, Ministry of Education, The First Hospital, Jilin University, Changchun, Jilin 130032, P.R. China; 3Department of Medical Laboratory Sciences, Haramaya University, Dire Dawa 3000, Ethiopia Received June 30, 2015; Accepted March 3, 2016 DOI: 10.3892/etm.2016.3424 Abstract. Cancer-associated genes serve a crucial role in patients with CRC. The results suggest that increased MCRS1 carcinogenesis. The present study aimed to investigate the and decreased MCRS2 expression appeared to be involved mRNA expression levels of microspherule protein 1 (MCRS1) in the pathogenesis of CRC. The present study provides and MCRS2 in colorectal cancer (CRC) and their associa- evidence suggesting that MCRS1 and MCRS2 may identify tion with clinical variables. The mRNA expression levels of CRC patients at a risk of disease relapse, and thus, may be MCRS1 and MCRS2 were assessed by semi-quantitative potential tools for monitoring disease activity and act as novel reverse transcription polymerase chain reaction in the tumor diagnostic markers in the treatment of CRC. and corresponding non-tumor tissues of 54 newly-diagnosed CRC patients, as well as in the normal colonic mucosa tissue of Introduction 19 age/gender‑matched healthy controls. Immunofluorescence was also employed to identify the expression of MCRS1 in Colorectal cancer (CRC) is one of the most prevalent malig- CRC tissues, while the concentration of serum carcinoem- nant tumors, with high incidence rate and mortality. -

DATASHEET USA: [email protected]
DATASHEET USA: [email protected] FOR IN VITRO RESEARCH USE ONLY Europe: [email protected] NOT FOR USE IN HUMANS OR ANIMALS China: [email protected] MCRS1 Polyclonal Anbody Catalog number: 11362-1-AP Background Size: 45 μg/150 μl MCRS1, also named INO80Q, MSP58 or p78, is a nucleolar protein expressed in the Source: Rabbit very early S phase. It modulates the transcripon repressor acvity of DAXX by Isotype: IgG recruing it to the nucleolus. MCRS1 is also present on polyribosomes of Synonyms: synaptoneurosome as a novel RNA-binding protein to acvate the rRNA MCRS1; 58 kDa microspherule transcripon. The forkhead-associated domain of MCRS1 is involved in associaon protein, ICP22BP, INO80 with centrosomal protein Nde1, comprising a component of the centrosome and complex subunit J, INO80Q, taking an essenal role in viability. Its isoform MCRS2 might be a linker between MCRS1, MCRS2, microspherule telomere maintenance and cell-cycle regulaon by interacng with LPTS/PinX1, a protein 1, MSP58, P78 telomerase-inhibitory protein. MCRS1 is detected by Western blot as a highly modified protein at 78 kDa and minor bands at about 62 and 55 kDa. Applications Tested applicaons: ELISA, WB Cited applicaons: WB Species specificity: Human,Mouse,Rat; other species not tested. Cited species: Human, mouse HeLa cells were subjected to SDS Caculated MCRS1 MW: 51 kDa PAGE followed by western blot with 11362-1-AP(MCRS1 anbody) at Observed MCRS1 MW: 55 kDa diluon of 1:500 Posive WB detected in HeLa cells, human brain ssue, human liver ssue, human spleen ssue, Jurkat cells, mouse spleen ssue, mouse uterus ssue, rat spleen ssue Recommended diluon: WB: 1:500-1:5000 Applicaon key: WB = Western blong, IHC = Immunohistochemistry, IF = Immunofluorescence, IP = Immunoprecipitaon Immunogen information Immunogen: Ag1904 GenBank accession number: BC011794 Gene ID (NCBI): 10445 Full name: Microspherule protein 1 Product information Purificaon method: Angen affinity purificaon Storage: PBS with 0.1% sodium azide and 50% glycerol pH 7.3. -

DNA Binding Features of Human POT1 a NONAMER 5Ј-TAGGGTTAG-3Ј MINIMAL BINDING SITE, SEQUENCE SPECIFICITY, and INTERNAL BINDING to MULTIMERIC SITES*
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 13, Issue of March 26, pp. 13241–13248, 2004 © 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. DNA Binding Features of Human POT1 A NONAMER 5Ј-TAGGGTTAG-3Ј MINIMAL BINDING SITE, SEQUENCE SPECIFICITY, AND INTERNAL BINDING TO MULTIMERIC SITES* Received for publication, November 10, 2003, and in revised form, January 7, 2004 Published, JBC Papers in Press, January 7, 2004, DOI 10.1074/jbc.M312309200 Diego Loayza‡§, Heather Parsons‡, Jill Donigian, Kristina Hoke¶, and Titia de Langeʈ From the Laboratory for Cell Biology and Genetics, The Rockefeller University, New York, New York 10021 The human telomeric protein POT1 is known to bind tankyrase 1 and 2, Tin2, PINX1, and POT1 are recruited to single-stranded telomeric DNA in vitro and to partici- telomeres by TRF1 (4). The TRF1 complex is involved in the pate in the regulation of telomere maintenance by te- regulation of telomere length by cis-inhibition of telomerase. In lomerase in vivo. We examined the in vitro DNA binding telomerase-positive cells, the overexpression of TRF1 leads to features of POT1. We report that deleting the oligosac- telomere shortening, and the expression of a dominant nega- charide/oligonucleotide-binding fold of POT1 abrogates tive form leads to telomere elongation (5). The relationship its DNA binding activity. The minimal binding site between TRF1 and telomerase regulation at the 3Ј telomere (MBS) for POT1 was found to be the telomeric nonamer terminus is poorly understood. 5 -TAGGGTTAG-3 , and the optimal substrate is The human single-stranded telomeric DNA-binding protein [TTAGGG]n (n > 2). -

Qt38n028mr Nosplash A3e1d84
! ""! ACKNOWLEDGEMENTS I dedicate this thesis to my parents who inspired me to become a scientist through invigorating scientific discussions at the dinner table even when I was too young to understand what the hippocampus was. They also prepared me for the ups and downs of science and supported me through all of these experiences. I would like to thank my advisor Dr. Elizabeth Blackburn and my thesis committee members Dr. Eric Verdin, and Dr. Emmanuelle Passegue. Liz created a nurturing and supportive environment for me to explore my own ideas, while at the same time teaching me how to love science, test my questions, and of course provide endless ways to think about telomeres and telomerase. Eric and Emmanuelle both gave specific critical advice about the proper experiments for T cells and both volunteered their lab members for further critical advice. I always felt inspired with a sense of direction after thesis committee meetings. The Blackburn lab is full of smart and dedicated scientists whom I am thankful for their support. Specifically Dr. Shang Li and Dr. Brad Stohr for their stimulating scientific debates and “arguments.” Dr. Jue Lin, Dana Smith, Kyle Lapham, Dr. Tet Matsuguchi, and Kyle Jay for their friendships and discussions about what my data could possibly mean. Dr. Eva Samal for teaching me molecular biology techniques and putting up with my late night lab exercises. Beth Cimini for her expertise with microscopy, FACs, singing, and most of all for being a caring and supportive friend. Finally, I would like to thank Dr. Imke Listerman, my scientific partner for most of the breast cancer experiments. -

A High-Density Map for Navigating the Human Polycomb Complexome
Resource A High-Density Map for Navigating the Human Polycomb Complexome Graphical Abstract Authors Simon Hauri, Federico Comoglio, Makiko Seimiya, ..., Renato Paro, Matthias Gstaiger, Christian Beisel Correspondence [email protected] (M.G.), [email protected] (C.B.) In Brief Polycomb group (PcG) proteins mediate gene silencing and epigenetic memory in higher eukaryotes. By systematically mapping the human PcG complexome, Hauri et al. resolve Polycomb subcomplexes at high resolution and identify two human PRC2 and two PR- DUB complexes. Furthermore, genomic profiling reveals segregation of PRC1 and PR-DUB target genes. Highlights Accession Numbers d 1,400 high-confidence interactions reveal the modular GSE51673 organization of human PcG proteins d Detailed dissection of PRC1 and PRC2 subcomplexes d Two human PR-DUB complexes contain the glycosyltransferase OGT1 d PR-DUB and PRC1 bind largely distinct sets of target genes Hauri et al., 2016, Cell Reports 17, 583–595 October 4, 2016 ª 2016 The Authors. http://dx.doi.org/10.1016/j.celrep.2016.08.096 Cell Reports Resource A High-Density Map for Navigating the Human Polycomb Complexome Simon Hauri,1,2,7,8 Federico Comoglio,3,7,9 Makiko Seimiya,3 Moritz Gerstung,3,10 Timo Glatter,1,11 Klaus Hansen,4 Ruedi Aebersold,1,5 Renato Paro,3,6 Matthias Gstaiger,1,2,* and Christian Beisel3,12,* 1Department of Biology, Institute of Molecular Systems Biology, ETH Zurich,€ 8093 Zurich,€ Switzerland 2Competence Center Personalized Medicine UZH/ETH, 8044 Zurich,€ Switzerland 3Department -

The Kinesin Spindle Protein Inhibitor Filanesib Enhances the Activity of Pomalidomide and Dexamethasone in Multiple Myeloma
Plasma Cell Disorders SUPPLEMENTARY APPENDIX The kinesin spindle protein inhibitor filanesib enhances the activity of pomalidomide and dexamethasone in multiple myeloma Susana Hernández-García, 1 Laura San-Segundo, 1 Lorena González-Méndez, 1 Luis A. Corchete, 1 Irena Misiewicz- Krzeminska, 1,2 Montserrat Martín-Sánchez, 1 Ana-Alicia López-Iglesias, 1 Esperanza Macarena Algarín, 1 Pedro Mogollón, 1 Andrea Díaz-Tejedor, 1 Teresa Paíno, 1 Brian Tunquist, 3 María-Victoria Mateos, 1 Norma C Gutiérrez, 1 Elena Díaz- Rodriguez, 1 Mercedes Garayoa 1* and Enrique M Ocio 1* 1Centro Investigación del Cáncer-IBMCC (CSIC-USAL) and Hospital Universitario-IBSAL, Salamanca, Spain; 2National Medicines Insti - tute, Warsaw, Poland and 3Array BioPharma, Boulder, Colorado, USA *MG and EMO contributed equally to this work ©2017 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol. 2017.168666 Received: March 13, 2017. Accepted: August 29, 2017. Pre-published: August 31, 2017. Correspondence: [email protected] MATERIAL AND METHODS Reagents and drugs. Filanesib (F) was provided by Array BioPharma Inc. (Boulder, CO, USA). Thalidomide (T), lenalidomide (L) and pomalidomide (P) were purchased from Selleckchem (Houston, TX, USA), dexamethasone (D) from Sigma-Aldrich (St Louis, MO, USA) and bortezomib from LC Laboratories (Woburn, MA, USA). Generic chemicals were acquired from Sigma Chemical Co., Roche Biochemicals (Mannheim, Germany), Merck & Co., Inc. (Darmstadt, Germany). MM cell lines, patient samples and cultures. Origin, authentication and in vitro growth conditions of human MM cell lines have already been characterized (17, 18). The study of drug activity in the presence of IL-6, IGF-1 or in co-culture with primary bone marrow mesenchymal stromal cells (BMSCs) or the human mesenchymal stromal cell line (hMSC–TERT) was performed as described previously (19, 20). -

Association Between the PINX1 and NAT2 Polymorphisms and Serum Lipid Levels
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 69), pp: 114081-114094 Research Paper Association between the PINX1 and NAT2 polymorphisms and serum lipid levels Qing-Hui Zhang1, Rui-Xing Yin1, Feng Huang1, De-Zhai Yang2, Wei-Xiong Lin2 and Shang-Ling Pan3 1Department of Cardiology, Institute of Cardiovascular Diseases, The First Affiliated Hospital, Guangxi Medical University, Nanning 530021, Guangxi, People’s Republic of China 2Department of Molecular Genetics, Medical Scientific Research Center, Guangxi Medical University, Nanning 530021, Guangxi, People’s Republic of China 3Department of Pathophysiology, School of Premedical Sciences, Guangxi Medical University, Nanning 530021, Guangxi, People’s Republic of China Correspondence to: Rui-Xing Yin, email: [email protected] Keywords: the PIN2/TERF1-interacting telomerase inhibitor 1; the N-acetyltransferase 2; single nucleotide polymorphism; lipids; environmental factor Received: September 27, 2017 Accepted: November 29, 2017 Published: December 09, 2017 Copyright: Zhang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Jing nationality is a relatively conservative and isolated minority in China. Little is known about the association of the PIN2/TERF1-interacting telomerase inhibitor 1 (PINX1) and N-acetyltransferase 2 (NAT2) single nucleotide polymorphisms (SNPs) and serum lipid levels in the Chinese populations. This study aimed to clarify the association of 6 SNPs of the PINX1 and NAT2 and serum lipid levels in two Chinese populations. Genotyping of the SNPs was performed in 1236 Han subjects and 1248 Jing participants.