Emmetropization in Arthropods: a New Vision Test in Several
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spectral Sensitivities of Wolf Spider Eyes
=ORNELL UNIVERSITY ~',lC.V~;AL ~ULLE(.~E DEF'ARY~,:ENT OF P~-I:~IOLOGY 1300 YORK AVE.~UE NEW YORK, N.Y. Spectral Sensitivities of Wolf Spider Eyes ROBERT D. DBVOE, RALPH J. W. SMALL, and JANIS E. ZVARGULIS From the Department of Physiology, The Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 ABSTRACT ERG's to spectral lights were recorded from all eyes of intact wolf spiders. Secondary eyes have maximum relative sensitivities at 505-510 nm which are unchanged by chromatic adaptations. Principal eyes have ultraviolet sensitivities which are 10 to 100 times greater at 380 nm than at 505 nm. How- ever, two animals' eyes initially had greater blue-green sensitivities, then in 7 to 10 wk dropped 4 to 6 log units in absolute sensitivity in the visible, less in the ultraviolet. Chromatic adaptations of both types of principal eyes hardly changed relative spectral sensitivities. Small decreases in relative sensitivity in the visible with orange adaptations were possibly retinomotor in origin. Second peaks in ERG waveforms were elicited from ultraviolet-adapted principal eyes by wavelengths 400 nm and longer, and from blue-, yellow-, and orange- adapted secondary eyes by wavelengths 580 nm and longer. The second peaks in waveforms were most likely responses of unilluminated eyes to scattered light. It is concluded that both principal and secondary eyes contain cells with a visual pigment absorbing maximally at 505-510 nm. The variable absolute and ultraviolet sensitivities of principal eyes may be due to a second pigment in the same cells or to an ultraviolet-absorbing accessory pigment which excites the 505 nm absorbing visual pigment by radiationless energy transfer. -

Arthropod IGF, Relaxin and Gonadulin, Putative Orthologs of Drosophila
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.11.088476; this version posted June 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Arthropod IGF, Relaxin and Gonadulin, putative 2 orthologs of Drosophila insulin-like peptides 6, 7 and 3 8, likely originated from an ancient gene triplication 4 5 6 Jan A. Veenstra1, 7 8 1 INCIA UMR 5287 CNRS, University of Bordeaux, Bordeaux, Pessac, France 9 10 Corresponding Author: 11 Jan A. Veenstra1 12 INCIA UMR 5287 CNRS, Université de Bordeaux, allée Geoffroy St Hillaire, CS 50023, 33 615 13 Pessac Cedex, France 14 Email address: [email protected] 15 16 Abstract 17 Background. Insects have several genes coding for insulin-like peptides and they have been 18 particularly well studied in Drosophila. Some of these hormones function as growth hormones 19 and are produced by the fat body and the brain. These act through a typical insulin receptor 20 tyrosine kinase. Two other Drosophila insulin-like hormones are either known or suspected to act 21 through a G-protein coupled receptor. Although insulin-related peptides are known from other 22 insect species, Drosophila insulin-like peptide 8, one that uses a G-protein coupled receptor, has 23 so far only been identified from Drosophila and other flies. However, its receptor is widespread 24 within arthropods and hence it should have orthologs. Such putative orthologs were recently 25 identified in decapods and have been called gonadulins. -

Historical, Landscape and Resource Influences on the Coccinellid Community in Missouri
HISTORICAL, LANDSCAPE AND RESOURCE INFLUENCES ON THE COCCINELLID COMMUNITY IN MISSOURI _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the requirements for the Degree Doctor of Philosophy _____________________________________________________ by LAUREN M. DIEPENBROCK Dr. Deborah Finke, Dissertation Supervisor MAY 2014 The undersigned, appointed by the Dean of the Graduate School, have examined the dissertation entitled: HISTORICAL, LANDSCAPE AND RESOURCE INFLUENCES ON THE COCCINELLID COMMUNITY IN MISSOURI Presented by Lauren M. Diepenbrock a candidate for the degree of Doctor of Philosophy and hereby certify that in their opinion is worthy of acceptance ________________________________________________ Dr. Deborah Finke, Dissertation Supervisor, Division of Plant Sciences ________________________________________________ Dr. Richard Houseman, Division of Plant Sciences ________________________________________________ Dr. Bruce Barrett, Division of Plant Sciences ________________________________________________ Dr. John Faaborg, Division of Biological Sciences ACKNOWLEDGEMENTS I would like to thank my Ph. D. advisor, Dr. Deborah Finke for the opportunity to pursue a doctoral degree in insect ecology and for her guidance and support throughout my time at the University of Missouri. I would also like to thank my graduate committee, Drs. Houseman, Barrett and Faaborg for their helpful advice during this academic journey. In addition to my graduate committee, I am grateful for the advice and opportunities that were made available to me by Dr. Rose-Marie Muzika, who introduced me to the Conservation Biology certificate program and all of the great researchers across the university who share my interests in biodiversity conservation. I will always be grateful to Dr. Jeanne Mihail for introducing me to Dr. -

Seeing Through Moving Eyes
bioRxiv preprint doi: https://doi.org/10.1101/083691; this version posted June 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Seeing through moving eyes - microsaccadic information sampling provides 2 Drosophila hyperacute vision 3 4 Mikko Juusola1,2*‡, An Dau2‡, Zhuoyi Song2‡, Narendra Solanki2, Diana Rien1,2, David Jaciuch2, 5 Sidhartha Dongre2, Florence Blanchard2, Gonzalo G. de Polavieja3, Roger C. Hardie4 and Jouni 6 Takalo2 7 8 1National Key laboratory of Cognitive Neuroscience and Learning, Beijing, Beijing Normal 9 University, Beijing 100875, China 10 2Department of Biomedical Science, University of Sheffield, Sheffield S10 T2N, UK 11 3Champalimaud Neuroscience Programme, Champalimaud Center for the Unknown, Lisbon, 12 Portugal 13 4Department of Physiology Development and Neuroscience, Cambridge University, Cambridge CB2 14 3EG, UK 15 16 *Correspondence to: [email protected] 17 ‡ Equal contribution 18 19 Small fly eyes should not see fine image details. Because flies exhibit saccadic visual behaviors 20 and their compound eyes have relatively few ommatidia (sampling points), their photoreceptors 21 would be expected to generate blurry and coarse retinal images of the world. Here we 22 demonstrate that Drosophila see the world far better than predicted from the classic theories. 23 By using electrophysiological, optical and behavioral assays, we found that R1-R6 24 photoreceptors’ encoding capacity in time is maximized to fast high-contrast bursts, which 25 resemble their light input during saccadic behaviors. Whilst over space, R1-R6s resolve moving 26 objects at saccadic speeds beyond the predicted motion-blur-limit. -

Introduction; Environment & Review of Eyes in Different Species
The Biological Vision System: Introduction; Environment & Review of Eyes in Different Species James T. Fulton https://neuronresearch.net/vision/ Abstract: Keywords: Biological, Human, Vision, phylogeny, vitamin A, Electrolytic Theory of the Neuron, liquid crystal, Activa, anatomy, histology, cytology PROCESSES IN BIOLOGICAL VISION: including, ELECTROCHEMISTRY OF THE NEURON Introduction 1- 1 1 Introduction, Phylogeny & Generic Forms 1 “Vision is the process of discovering from images what is present in the world, and where it is” (Marr, 1985) ***When encountering a citation to a Section number in the following material, the first numeric is a chapter number. All cited chapters can be found at https://neuronresearch.net/vision/document.htm *** 1.1 Introduction While the material in this work is designed for the graduate student undertaking independent study of the vision sensory modality of the biological system, with a certain amount of mathematical sophistication on the part of the reader, the major emphasis is on specific models down to specific circuits used within the neuron. The Chapters are written to stand-alone as much as possible following the block diagram in Section 1.5. However, this requires frequent cross-references to other Chapters as the analyses proceed. The results can be followed by anyone with a college degree in Science. However, to replicate the (photon) Excitation/De-excitation Equation, a background in differential equations and integration-by-parts is required. Some background in semiconductor physics is necessary to understand how the active element within a neuron operates and the unique character of liquid-crystalline water (the backbone of the neural system). The level of sophistication in the animal vision system is quite remarkable. -

University of Groningen Colour in the Eyes of Insects Stavenga, D.G
University of Groningen Colour in the eyes of insects Stavenga, D.G. Published in: Journal of Comparative Physiology A; Sensory Neural, and Behavioral Physiology DOI: 10.1007/s00359-002-0307-9 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2002 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Stavenga, D. G. (2002). Colour in the eyes of insects. Journal of Comparative Physiology A; Sensory Neural, and Behavioral Physiology, 188(5), 337-348. https://doi.org/10.1007/s00359-002-0307-9 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 26-09-2021 J Comp Physiol A (2002) 188: 337–348 DOI 10.1007/s00359-002-0307-9 REVIEW D.G. Stavenga Colour in the eyes of insects Accepted: 15 March 2002 / Published online: 13 April 2002 Ó Springer-Verlag 2002 Abstract Many insect species have darkly coloured provide the input for the visual neuropiles, which eyes, but distinct colours or patterns are frequently process the light signals to detect motion, colours, or featured. -

(Coleoptera: Coccinellidae) of the Atlantic Maritime Ecozone
Chapter 21 Ladybird beetles (Coleoptera: Coccinellidae) of the Atlantic Maritime Ecozone Christopher G. Majka and David B. McCorquodale Abstract: The Atlantic Maritime Ecozone Coccinellidae (lady beetles) are a conspicuous component of the region’s beetle fauna. Fifty-one species have been recorded, six of which are introduced. In addition, there are records of intentional and inadvertent introductions of 10 species that have not persisted. In this treatment, we discuss the general biology of the Coccinellidae, the fauna of the Atlantic Maritime Ecozone, and the history of collecting efforts for the group. The distri- bution of species (in varying degrees of detail) is examined with a consideration of the differences between provinces. Attention is paid to the zoogeographic factors that influence distribution, including the role of geography, latitude, and the isolation of island faunas. The ecology, distribution, and systematics of individual species are discussed with a consider- ation of the role of both the geological history in shaping the distribution of native species, and human history and agency in the distribution of introduced species. We also consider the impact of introduced species on native faunas and examine the composition of the fauna in terms of global and continental zoogeographic patterns. Résumé : Les coccinellidés (coccinelles) de l’écozone maritime de l’Atlantique sont une composante remarquable de la faune de coléoptères de la région. Cinquante-et-une espèces ont été répertoriées, parmi lesquelles six ont été introduites. Il existe en outre des témoignages d’introductions intentionnelles et non intentionnelles de dix espèces qui n’ont pas per- sisté. Dans ce document, nous abordons la biologie des coccinellidés en général, la faune qui occupe l’écozone maritime de l’Atlantique en particulier, et l’historique des efforts de collecte pour ce groupe. -

Glossary Animal Physiology Circulatory System (See Also Human Biology 1)
1 Glossary Animal Physiology Circulatory System (see also Human Biology 1) Aneurism: Localized dilatation of the artery wall due to the rupture of collagen sheaths. Arteriosclerosis: A disease marked by an increase in thickness and a reduction in elasticity of the arterial wall; SMC, smooth muscle cells can (due to an increase in Na-intake or permanent stress related factors) be stimulated to increase deposition of SMC in the media surrounding the artery resulting in a decreased lumen available for the blood to be transported, hence rising the blood pressure, which itself signalizes to the SMC that more cells to be deposited to resist the increase pressure until little lumen is left over, leading for example to heart attack. Arterial System: The branching vessels that are thick, elastic, and muscular, with the following functions: • act as a conduit for blood between the heart and capillaries • act as pressure reservoir for forcing blood into small-diameter arterioles • dampen heart -related oscillations of pressure and flow, results in an even flow of blood into capillaries • control distribution of blood to different capillary networks via selective constriction of the terminal branches of the arterial tree. Atria: A chamber that gives entrance to another structure or organ; usually used to refer to the atrium of the heart. Baroreceptor: Sensory nerve ending, stimulated by changes in pressure, as those in the walls of blood vessels. Blood: The fluid (composed of 45% solid compounds and 55% liquid) circulated by the heart in a vertebrate, carrying oxygen, nutrients, hormones, defensive proteins (albumins and globulins, fibronigen etc.), throughout the body and waste materials to excretory organs; it is functionally similar in invertebrates. -
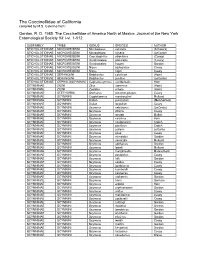
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Arthropod Genomics Symposium (Ags) Thank You to Our Sponsors
11th Annual ARTHROPOD GENOMICS SYMPOSIUM (AGS) THANK YOU TO OUR SPONSORS Carl R. Woese Institute for Genomic Biology Department of Entomology School of Integrative Biology 11th Annual ARTHROPOD GENOMICS SYMPOSIUM (AGS) WELCOME THANK YOU TO OUR SPONSORS 11TH ANNUAL ARTHROPOD GENOMICS SYMPOSIUM (AGS) Welcome to the 11th Annual Arthropod Genomics Symposium. The University of Illinois at Urbana-Champaign is pleased to host the Arthropod Genomics Symposium, Thursday to Saturday, June 7-9, 2018. It has been 18 years since the sequencing of the genome of Drosophila melanogaster, and over three hundred arthropod genomes have been sequenced since then. The Arthropod Genomics Symposium is an opportunity to reflect on recent progress and explore future directions. We have planned an array of sessions that reflect the diversity of arthropod genomics. We hope you will join us for a productive exchange of ideas and viewpoints, and we look forward to seeing you here. —Hugh Robertson and Gene Robinson, co-chairs AGENDA Alice Campbell Alumni Center 601 South Lincoln Avenue, Urbana, IL 61801 Thursday, June 7, 2018 12:30 PM - 1:00 PM Registration opens for Pre-symposium Workshop registrants 1:00 PM - 5:00 PM Pre-symposium Workshop Monica Poelchau NAL/USDA Maryland Monica Poelchau NAL/USDA Maryland Robert Waterhouse University of Lausanne 5:00 PM Registration opens and Reception 7:00 PM Welcome May Berenbaum University of Illinois Plenary lecture Michael Lynch Arizona State University “The 5000 Daphnia Genome Project” Friday, June 8, 2018 7:30 AM Coffee/Tea -

Biological Sciences
A Comprehensive Book on Environmentalism Table of Contents Chapter 1 - Introduction to Environmentalism Chapter 2 - Environmental Movement Chapter 3 - Conservation Movement Chapter 4 - Green Politics Chapter 5 - Environmental Movement in the United States Chapter 6 - Environmental Movement in New Zealand & Australia Chapter 7 - Free-Market Environmentalism Chapter 8 - Evangelical Environmentalism Chapter 9 -WT Timeline of History of Environmentalism _____________________ WORLD TECHNOLOGIES _____________________ A Comprehensive Book on Enzymes Table of Contents Chapter 1 - Introduction to Enzyme Chapter 2 - Cofactors Chapter 3 - Enzyme Kinetics Chapter 4 - Enzyme Inhibitor Chapter 5 - Enzymes Assay and Substrate WT _____________________ WORLD TECHNOLOGIES _____________________ A Comprehensive Introduction to Bioenergy Table of Contents Chapter 1 - Bioenergy Chapter 2 - Biomass Chapter 3 - Bioconversion of Biomass to Mixed Alcohol Fuels Chapter 4 - Thermal Depolymerization Chapter 5 - Wood Fuel Chapter 6 - Biomass Heating System Chapter 7 - Vegetable Oil Fuel Chapter 8 - Methanol Fuel Chapter 9 - Cellulosic Ethanol Chapter 10 - Butanol Fuel Chapter 11 - Algae Fuel Chapter 12 - Waste-to-energy and Renewable Fuels Chapter 13 WT- Food vs. Fuel _____________________ WORLD TECHNOLOGIES _____________________ A Comprehensive Introduction to Botany Table of Contents Chapter 1 - Botany Chapter 2 - History of Botany Chapter 3 - Paleobotany Chapter 4 - Flora Chapter 5 - Adventitiousness and Ampelography Chapter 6 - Chimera (Plant) and Evergreen Chapter -

Insect-Inspired Vision for Autonomous Vehicles Julien Serres, Stéphane Viollet
Insect-inspired vision for autonomous vehicles Julien Serres, Stéphane Viollet To cite this version: Julien Serres, Stéphane Viollet. Insect-inspired vision for autonomous vehicles. Current Opinion in Insect Science, Elsevier, 2018, 10.1016/j.cois.2018.09.005. hal-01882712 HAL Id: hal-01882712 https://hal-amu.archives-ouvertes.fr/hal-01882712 Submitted on 27 Sep 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Insect-inspired vision for autonomous vehicles Julien R. Serres1 and Stéphane Viollet1 1Aix Marseille University, CNRS, ISM, Marseille, France September 10, 2018 Highlights: • Compound eyes are an endless source of inspiration for developing visual sensors • Visual stabilization of robot’s flight attitude controlled by artificial ocelli • Ultraviolet celestial cue-based navigation works efficiently under all weather conditions • Combining blurry vision with retinal micro-movements makes robots’ vi- sual tracking hyperacute Abstract: Flying insects are being studied these days as if they were agile micro air vehicles fitted with smart sensors, requiring very few brain resources. The findings obtained on these natural fliers have proved to be extremely valuable when it comes to designing compact low-weight artificial optical sensors capable of performing visual processing tasks robustly under various environmental conditions (light, clouds, contrast).