Historical, Landscape and Resource Influences on the Coccinellid Community in Missouri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cold Tolerance of the Predatory Ladybird Cryptolaemus Montrouzieri
BioControl (2015) 60:199–207 DOI 10.1007/s10526-014-9630-7 Cold tolerance of the predatory ladybird Cryptolaemus montrouzieri Sara Maes • Jean-Claude Gre´goire • Patrick De Clercq Received: 21 May 2014 / Accepted: 15 October 2014 / Published online: 29 October 2014 Ó International Organization for Biological Control (IOBC) 2014 Abstract The effect of low temperature acclimation throughout development and in the first week of their and diet on the supercooling point (SCP, the temperature adult life. Also food source had a significant effect on the at which the insect’s body fluids freeze) and lethal time freezing temperature of C. montrouzieri:theSCPof (LTime, time required to kill 50 % of the population at a ladybirds fed the citrus mealybug, Planococcus citri temperature of 5 °C) of the mealybug destroyer, (Risso)(Hemiptera: Pseudococcidae), was 1.6 °C Cryptolaemus montrouzieri Mulsant (Coleoptera: Coc- higher than the value of -17.2 °C observed for cinellidae), was assessed in the laboratory. The SCP of ladybirds provided with eggs of the flour moth Ephestia acclimated adult ladybirds which were allowed to kuehniella Zeller (Lepidoptera: Pyralidae). However, complete development to adulthood at 18 °Canda neither cold acclimation nor diet had a significant effect 8:16(L:D)h photoperiod, or at 25 °C and a 16:8(L:D)h on the lethal times of C. montrouzieri. Overall, the time photoperiod, and which were subsequently kept at required to kill 50 % of the population at a temperature 10 °C and a 12:12(L:D)h photoperiod for seven days, of 5 °C ranged from 12.8 days for ladybirds fed P. -

Arthropod IGF, Relaxin and Gonadulin, Putative Orthologs of Drosophila
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.11.088476; this version posted June 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Arthropod IGF, Relaxin and Gonadulin, putative 2 orthologs of Drosophila insulin-like peptides 6, 7 and 3 8, likely originated from an ancient gene triplication 4 5 6 Jan A. Veenstra1, 7 8 1 INCIA UMR 5287 CNRS, University of Bordeaux, Bordeaux, Pessac, France 9 10 Corresponding Author: 11 Jan A. Veenstra1 12 INCIA UMR 5287 CNRS, Université de Bordeaux, allée Geoffroy St Hillaire, CS 50023, 33 615 13 Pessac Cedex, France 14 Email address: [email protected] 15 16 Abstract 17 Background. Insects have several genes coding for insulin-like peptides and they have been 18 particularly well studied in Drosophila. Some of these hormones function as growth hormones 19 and are produced by the fat body and the brain. These act through a typical insulin receptor 20 tyrosine kinase. Two other Drosophila insulin-like hormones are either known or suspected to act 21 through a G-protein coupled receptor. Although insulin-related peptides are known from other 22 insect species, Drosophila insulin-like peptide 8, one that uses a G-protein coupled receptor, has 23 so far only been identified from Drosophila and other flies. However, its receptor is widespread 24 within arthropods and hence it should have orthologs. Such putative orthologs were recently 25 identified in decapods and have been called gonadulins. -

(Coleoptera: Coccinellidae) of the Atlantic Maritime Ecozone
Chapter 21 Ladybird beetles (Coleoptera: Coccinellidae) of the Atlantic Maritime Ecozone Christopher G. Majka and David B. McCorquodale Abstract: The Atlantic Maritime Ecozone Coccinellidae (lady beetles) are a conspicuous component of the region’s beetle fauna. Fifty-one species have been recorded, six of which are introduced. In addition, there are records of intentional and inadvertent introductions of 10 species that have not persisted. In this treatment, we discuss the general biology of the Coccinellidae, the fauna of the Atlantic Maritime Ecozone, and the history of collecting efforts for the group. The distri- bution of species (in varying degrees of detail) is examined with a consideration of the differences between provinces. Attention is paid to the zoogeographic factors that influence distribution, including the role of geography, latitude, and the isolation of island faunas. The ecology, distribution, and systematics of individual species are discussed with a consider- ation of the role of both the geological history in shaping the distribution of native species, and human history and agency in the distribution of introduced species. We also consider the impact of introduced species on native faunas and examine the composition of the fauna in terms of global and continental zoogeographic patterns. Résumé : Les coccinellidés (coccinelles) de l’écozone maritime de l’Atlantique sont une composante remarquable de la faune de coléoptères de la région. Cinquante-et-une espèces ont été répertoriées, parmi lesquelles six ont été introduites. Il existe en outre des témoignages d’introductions intentionnelles et non intentionnelles de dix espèces qui n’ont pas per- sisté. Dans ce document, nous abordons la biologie des coccinellidés en général, la faune qui occupe l’écozone maritime de l’Atlantique en particulier, et l’historique des efforts de collecte pour ce groupe. -

Status Report on Arthropod Predators of the Asian Citrus Psyllid in Mexico
Status report on arthropod predators of the Asian Citrus Psyllid in Mexico Esteban Rodríguez-Leyva Colegio de Postgraduados, Montecillo, Texcoco, MEXICO. Importance of Citrus in Mexico 525 000 ha 23 States Citrus production 6.7 million ton/year 68% Orange, 20% Mexican lime, 5% Persian lime 2.5% Grapefruit , 2.5% Tangerine The insect problem Diaphorina citri in Mexico (www.senasica.gob.mx) Searching for natural enemies of ACP on citrus trees and orange jessamine Coccinellidae Azya orbigera Brachiacantha decora Chilocorus cacti Cycloneda sanguinea Harmonia axyridis Hippodamia convergens Olla v-nigrum López- Arroyo et al. 2008 Marín 2009, personal com. Sánchez-González & Arredondo-Bernal 2009, personal com. Gaona-García et al. 2009 Chrysopidae Chrysoperla rufilabris C. comanche C. externa Ceraeochrysa sp. nr. cincta C. valida López- Arroyo et al. 2008 Cortez-Mondaca et al. 2008 Syrphidae Allographa obliqua Pseudodoros clavatus Toxomerus marginatus Toxomerus politus Miscelaneous predators of ACP Spiders, Wasps (Vespidae), Ants (Ponerinae) López- Arroyo et al. 2008 Sánchez-González & Arredondo-Bernal 2009, personal com. Abundance of ladybirds (Coccinellidae) predators on citrus trees, at Nuevo Leon (northeast), Mexico. (Insect net, two sides of each mature tree, n=30). López-Arroyo, 2010, personal com. Abundance of lacewings (Chrysopidae) predators on citrus trees, at Nuevo Leon (northeast), Mexico. (Insect net two sides of each mature tree, n=30). López-Arroyo, 2010, personal com. Abundance of lacewings (Chrysopidae) predators on citrus trees, at Nuevo Leon (northeast), Mexico. (Insect net two sides of each mature tree, n=30). López-Arroyo, 2010, personal com. Abundance of predators (specially C. rufilabris) on Diaphorina citri on citrus trees at Nuevo Leon (northeast), Mexico. -

Biological Studies and Evaluation of Scymnus Coniferarum Crotch, a Predator of Hemlock Woolly Adelgid from Western North America
Biological studies and evaluation of Scymnus coniferarum Crotch, a predator of hemlock woolly adelgid from western North America Molly Norton Darr Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Entomology Scott M. Salom, Chair Timothy J. Kring Michael E. Montgomery James P. Parkman Paul E. Marek May 1, 2017 Blacksburg, VA Keywords: Biological control, Hemlock woolly adelgid, Adelges tsugae, Scymnus coniferarum, Tsuga heterophylla, Tsuga canadensis Biological studies and evaluation of Scymnus coniferarum Crotch, a predator of hemlock woolly adelgid from western North America Molly Norton Darr ABSTRACT (ACADEMIC) The hemlock woolly adelgid (HWA), Adelges tsugae Annand, is an invasive pest of eastern hemlock, Tsuga canadensis (L.) Carriere and Carolina hemlock Tsuga caroliniana Englem. in the eastern United States. A newly reported beetle predator for HWA, Scymnus (Pullus) coniferarum Crotch (Coleoptera: Cocinellidae) preys on the pest in the western United States, and was approved for release in the eastern United States for the control of HWA. This research investigated the viability of S. coniferarum as a biological control agent of A. tsugae in the eastern United States, as well as the ecological dynamics between S. coniferarum and host prey species in its native range of western North America. In objective one, S. coniferarum predation, reproductive potential, and survival were evaluated in field-cages on adelgid infested T. canadensis in southwestern Virginia. Adult S. coniferarum fed on both generations and all life stages of A. tsugae at rates comparable to other adelgid-specific predators, and survived for extended periods of time in the field. -
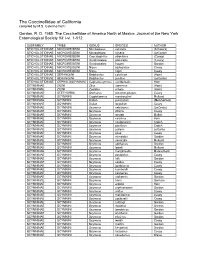
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Review of Clitostethus Weise, Parastethorus Pang Et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/327149552 Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan Article in Oriental insects · August 2018 DOI: 10.1080/00305316.2018.1492987 CITATIONS READS 0 20 4 authors, including: Zafar Iqbal Muhammad Farooq Nasir PMAS - Arid Agriculture University 7 PUBLICATIONS 0 CITATIONS 21 PUBLICATIONS 56 CITATIONS SEE PROFILE SEE PROFILE Imran Bodlah PMAS - Arid Agriculture University 69 PUBLICATIONS 119 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Faunastic study of Stratiomyidae (Diptera) from Pakistan View project termite cibtrol View project All content following this page was uploaded by Imran Bodlah on 28 August 2018. The user has requested enhancement of the downloaded file. Oriental Insects ISSN: 0030-5316 (Print) 2157-8745 (Online) Journal homepage: http://www.tandfonline.com/loi/toin20 Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan Zafar Iqbal, Muhammad Farooq Nasir, Imran Bodlah & Karol Szawaryn To cite this article: Zafar Iqbal, Muhammad Farooq Nasir, Imran Bodlah & Karol Szawaryn (2018): Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan, Oriental Insects, DOI: 10.1080/00305316.2018.1492987 To link to this article: https://doi.org/10.1080/00305316.2018.1492987 Published -

Arthropod Genomics Symposium (Ags) Thank You to Our Sponsors
11th Annual ARTHROPOD GENOMICS SYMPOSIUM (AGS) THANK YOU TO OUR SPONSORS Carl R. Woese Institute for Genomic Biology Department of Entomology School of Integrative Biology 11th Annual ARTHROPOD GENOMICS SYMPOSIUM (AGS) WELCOME THANK YOU TO OUR SPONSORS 11TH ANNUAL ARTHROPOD GENOMICS SYMPOSIUM (AGS) Welcome to the 11th Annual Arthropod Genomics Symposium. The University of Illinois at Urbana-Champaign is pleased to host the Arthropod Genomics Symposium, Thursday to Saturday, June 7-9, 2018. It has been 18 years since the sequencing of the genome of Drosophila melanogaster, and over three hundred arthropod genomes have been sequenced since then. The Arthropod Genomics Symposium is an opportunity to reflect on recent progress and explore future directions. We have planned an array of sessions that reflect the diversity of arthropod genomics. We hope you will join us for a productive exchange of ideas and viewpoints, and we look forward to seeing you here. —Hugh Robertson and Gene Robinson, co-chairs AGENDA Alice Campbell Alumni Center 601 South Lincoln Avenue, Urbana, IL 61801 Thursday, June 7, 2018 12:30 PM - 1:00 PM Registration opens for Pre-symposium Workshop registrants 1:00 PM - 5:00 PM Pre-symposium Workshop Monica Poelchau NAL/USDA Maryland Monica Poelchau NAL/USDA Maryland Robert Waterhouse University of Lausanne 5:00 PM Registration opens and Reception 7:00 PM Welcome May Berenbaum University of Illinois Plenary lecture Michael Lynch Arizona State University “The 5000 Daphnia Genome Project” Friday, June 8, 2018 7:30 AM Coffee/Tea -

Intraguild Interactions Between the Mealybug Predators Cryptolaemus Montrouzieri and Chrysoperla Carnea
insects Article Intraguild Interactions between the Mealybug Predators Cryptolaemus montrouzieri and Chrysoperla carnea Laura Golsteyn 1, Hana Mertens 1, Joachim Audenaert 2, Ruth Verhoeven 2, Bruno Gobin 2 and Patrick De Clercq 1,* 1 Department of Plants and Crops, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium; [email protected] (L.G.); [email protected] (H.M.) 2 PCS—Ornamental Plant Research, Schaessestraat 18, B-9070 Destelbergen, Belgium; [email protected] (J.A.); [email protected] (R.V.); [email protected] (B.G.) * Correspondence: [email protected]; Tel.: +32-92-646-158 Simple Summary: The ladybird Cryptolaemus montrouzieri is a widely commercialized biological control agent of mealybugs. The green lacewing Chrysoperla carnea is mainly released for aphid control, but also attacks mealybugs. Both species have shown potential to control various economically important species of mealybug pests of greenhouse crops. As these predators may be simultaneously present in a crop, the risk of negative interactions between both predators was evaluated in this laboratory study. Individuals of different life stages of either predator were placed together in petri dish arenas and predation was recorded. Attacks between individuals of both species were frequently observed, with lacewing larvae being the dominant predators in most combinations. When mealybug nymphs or lepidopteran eggs were added to the arena, the incidence of attacks between the predators was greatly diminished. The relevance of these observations for the use of the predators in the biological control of greenhouse pests is discussed. Citation: Golsteyn, L.; Mertens, H.; Audenaert, J.; Verhoeven, R.; Gobin, Abstract: The ladybird Cryptolaemus montrouzieri and the green lacewing Chrysoperla carnea have B.; De Clercq, P. -

Emmetropization in Arthropods: a New Vision Test in Several
Emmetropization in arthropods: A new vision test in several arthropods suggests visual input may not be necessary to establish correct focusing A thesis submitted to the Graduate School of The University of Cincinnati (Department of Biological Sciences) In partial fulfillment of the requirements for the degree of Master of Science In the Department of Biological Sciences of the College of Arts and Sciences July 2019 By Madeline Owens B.S. Biological Sciences, University of Cincinnati (2016) Committee Chair: Dr. Elke K. Buschbeck, Ph.D. This work will be submitted to The Journal of Experimental Biology Authors: Madeline Owens, Elke K. Buschbeck ABSTRACT The visual systems of vertebrates and invertebrates alike must be able to achieve and maintain emmetropia, a state of correct focusing, in order to effectively execute visually guided behavior. Vertebrate studies, as well as a study in squid, have revealed that for them correct focusing is accomplished through a combination of gene regulation during early development, and homeostatic visual input from the environment that fine-tunes the eye. While eye growth towards emmetropia has been long researched in vertebrates, it is largely unknown how it is established in arthropods. To address this question, we built a micro-ophthalmoscope that allows us to directly measure how a lens projects an image onto the retina in the eyes of small, live arthropods, and to compare the eyes of different light-reared and dark-reared arthropods. Surprisingly, and in sharp contrast to vertebrates, our data on a diverse set of arthropods suggests that visual input in arthropods may not be necessary at least for the initial development of emmetropic eyes. -

Endemics Versus Newcomers: the Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria
insects Article Endemics Versus Newcomers: The Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria Jerzy Romanowski 1,* , Piotr Ceryngier 1 , Jaroslav Vetrovec˘ 2, Marta Piotrowska 3 and Karol Szawaryn 4 1 Institute of Biological Sciences, Cardinal Stefan Wyszy´nskiUniversity, Wóycickiego 1/3, 01-938 Warsaw, Poland; [email protected] 2 Buzulucká, 1105 Hradec Králové, Czech Republic; [email protected] 3 Faculty of Biology and Environmental Sciences UKSW, ul. Wóycickiego 1/3, PL-01-938 Warsaw, Poland; [email protected] 4 Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, 00-679 Warsaw, Poland; [email protected] * Correspondence: [email protected] Received: 18 August 2020; Accepted: 14 September 2020; Published: 18 September 2020 Simple Summary: Many plants and animals that live in the Canary Islands belong to the so-called endemic species, i.e., they do not occur outside of this particular region. Several other species have a slightly wider geographical distribution, apart from the Canaries, which also includes some islands of the nearby archipelagos, such as Madeira or the Azores, or the northwestern periphery of Africa. Here, we call such species subendemics. However, the Canary Islands have recently been colonized by a substantial number of immigrants from more or less remote areas. In this paper, based on our field survey and previously published data, we analyzed the fauna of the ladybird beetles (Coccinellidae) of Gran Canaria, one of the central islands of the archipelago. Among 42 ladybird beetle species so far recorded on this island, 17 (40%) are endemics and subendemics, and 21 (50%) probably arrived in Gran Canaria relatively recently, i.e., in the 20th and 21st century. -

National Program 304 – Crop Protection and Quarantine
APPENDIX 1 National Program 304 – Crop Protection and Quarantine ACCOMPLISHMENT REPORT 2007 – 2012 Current Research Projects in National Program 304* SYSTEMATICS 1245-22000-262-00D SYSTEMATICS OF FLIES OF AGRICULTURAL AND ENVIRONMENTAL IMPORTANCE; Allen Norrbom (P), Sonja Jean Scheffer, and Norman E. Woodley; Beltsville, Maryland. 1245-22000-263-00D SYSTEMATICS OF BEETLES IMPORTANT TO AGRICULTURE, LANDSCAPE PLANTS, AND BIOLOGICAL CONTROL; Steven W. Lingafelter (P), Alexander Konstantinov, and Natalie Vandenberg; Washington, D.C. 1245-22000-264-00D SYSTEMATICS OF LEPIDOPTERA: INVASIVE SPECIES, PESTS, AND BIOLOGICAL CONTROL AGENTS; John W. Brown (P), Maria A. Solis, and Michael G. Pogue; Washington, D.C. 1245-22000-265-00D SYSTEMATICS OF PARASITIC AND HERBIVOROUS WASPS OF AGRICULTURAL IMPORTANCE; Robert R. Kula (P), Matthew Buffington, and Michael W. Gates; Washington, D.C. 1245-22000-266-00D MITE SYSTEMATICS AND ARTHROPOD DIAGNOSTICS WITH EMPHASIS ON INVASIVE SPECIES; Ronald Ochoa (P); Washington, D.C. 1245-22000-267-00D SYSTEMATICS OF HEMIPTERA AND RELATED GROUPS: PLANT PESTS, PREDATORS, AND DISEASE VECTORS; Thomas J. Henry (P), Stuart H. McKamey, and Gary L. Miller; Washington, D.C. INSECTS 0101-88888-040-00D OFFICE OF PEST MANAGEMENT; Sheryl Kunickis (P); Washington, D.C. 0212-22000-024-00D DISCOVERY, BIOLOGY AND ECOLOGY OF NATURAL ENEMIES OF INSECT PESTS OF CROP AND URBAN AND NATURAL ECOSYSTEMS; Livy H. Williams III (P) and Kim Hoelmer; Montpellier, France. * Because of the nature of their research, many NP 304 projects contribute to multiple Problem Statements, so for the sake of clarity they have been grouped by focus area. For the sake of consistency, projects are listed and organized in Appendix 1 and 2 according to the ARS project number used to track projects in the Agency’s internal database.