Cold Tolerance of the Predatory Ladybird Cryptolaemus Montrouzieri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical, Landscape and Resource Influences on the Coccinellid Community in Missouri
HISTORICAL, LANDSCAPE AND RESOURCE INFLUENCES ON THE COCCINELLID COMMUNITY IN MISSOURI _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the requirements for the Degree Doctor of Philosophy _____________________________________________________ by LAUREN M. DIEPENBROCK Dr. Deborah Finke, Dissertation Supervisor MAY 2014 The undersigned, appointed by the Dean of the Graduate School, have examined the dissertation entitled: HISTORICAL, LANDSCAPE AND RESOURCE INFLUENCES ON THE COCCINELLID COMMUNITY IN MISSOURI Presented by Lauren M. Diepenbrock a candidate for the degree of Doctor of Philosophy and hereby certify that in their opinion is worthy of acceptance ________________________________________________ Dr. Deborah Finke, Dissertation Supervisor, Division of Plant Sciences ________________________________________________ Dr. Richard Houseman, Division of Plant Sciences ________________________________________________ Dr. Bruce Barrett, Division of Plant Sciences ________________________________________________ Dr. John Faaborg, Division of Biological Sciences ACKNOWLEDGEMENTS I would like to thank my Ph. D. advisor, Dr. Deborah Finke for the opportunity to pursue a doctoral degree in insect ecology and for her guidance and support throughout my time at the University of Missouri. I would also like to thank my graduate committee, Drs. Houseman, Barrett and Faaborg for their helpful advice during this academic journey. In addition to my graduate committee, I am grateful for the advice and opportunities that were made available to me by Dr. Rose-Marie Muzika, who introduced me to the Conservation Biology certificate program and all of the great researchers across the university who share my interests in biodiversity conservation. I will always be grateful to Dr. Jeanne Mihail for introducing me to Dr. -

Biological Studies and Evaluation of Scymnus Coniferarum Crotch, a Predator of Hemlock Woolly Adelgid from Western North America
Biological studies and evaluation of Scymnus coniferarum Crotch, a predator of hemlock woolly adelgid from western North America Molly Norton Darr Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Entomology Scott M. Salom, Chair Timothy J. Kring Michael E. Montgomery James P. Parkman Paul E. Marek May 1, 2017 Blacksburg, VA Keywords: Biological control, Hemlock woolly adelgid, Adelges tsugae, Scymnus coniferarum, Tsuga heterophylla, Tsuga canadensis Biological studies and evaluation of Scymnus coniferarum Crotch, a predator of hemlock woolly adelgid from western North America Molly Norton Darr ABSTRACT (ACADEMIC) The hemlock woolly adelgid (HWA), Adelges tsugae Annand, is an invasive pest of eastern hemlock, Tsuga canadensis (L.) Carriere and Carolina hemlock Tsuga caroliniana Englem. in the eastern United States. A newly reported beetle predator for HWA, Scymnus (Pullus) coniferarum Crotch (Coleoptera: Cocinellidae) preys on the pest in the western United States, and was approved for release in the eastern United States for the control of HWA. This research investigated the viability of S. coniferarum as a biological control agent of A. tsugae in the eastern United States, as well as the ecological dynamics between S. coniferarum and host prey species in its native range of western North America. In objective one, S. coniferarum predation, reproductive potential, and survival were evaluated in field-cages on adelgid infested T. canadensis in southwestern Virginia. Adult S. coniferarum fed on both generations and all life stages of A. tsugae at rates comparable to other adelgid-specific predators, and survived for extended periods of time in the field. -
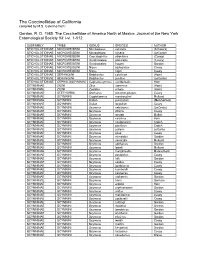
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Review of Clitostethus Weise, Parastethorus Pang Et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/327149552 Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan Article in Oriental insects · August 2018 DOI: 10.1080/00305316.2018.1492987 CITATIONS READS 0 20 4 authors, including: Zafar Iqbal Muhammad Farooq Nasir PMAS - Arid Agriculture University 7 PUBLICATIONS 0 CITATIONS 21 PUBLICATIONS 56 CITATIONS SEE PROFILE SEE PROFILE Imran Bodlah PMAS - Arid Agriculture University 69 PUBLICATIONS 119 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Faunastic study of Stratiomyidae (Diptera) from Pakistan View project termite cibtrol View project All content following this page was uploaded by Imran Bodlah on 28 August 2018. The user has requested enhancement of the downloaded file. Oriental Insects ISSN: 0030-5316 (Print) 2157-8745 (Online) Journal homepage: http://www.tandfonline.com/loi/toin20 Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan Zafar Iqbal, Muhammad Farooq Nasir, Imran Bodlah & Karol Szawaryn To cite this article: Zafar Iqbal, Muhammad Farooq Nasir, Imran Bodlah & Karol Szawaryn (2018): Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan, Oriental Insects, DOI: 10.1080/00305316.2018.1492987 To link to this article: https://doi.org/10.1080/00305316.2018.1492987 Published -

Intraguild Interactions Between the Mealybug Predators Cryptolaemus Montrouzieri and Chrysoperla Carnea
insects Article Intraguild Interactions between the Mealybug Predators Cryptolaemus montrouzieri and Chrysoperla carnea Laura Golsteyn 1, Hana Mertens 1, Joachim Audenaert 2, Ruth Verhoeven 2, Bruno Gobin 2 and Patrick De Clercq 1,* 1 Department of Plants and Crops, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium; [email protected] (L.G.); [email protected] (H.M.) 2 PCS—Ornamental Plant Research, Schaessestraat 18, B-9070 Destelbergen, Belgium; [email protected] (J.A.); [email protected] (R.V.); [email protected] (B.G.) * Correspondence: [email protected]; Tel.: +32-92-646-158 Simple Summary: The ladybird Cryptolaemus montrouzieri is a widely commercialized biological control agent of mealybugs. The green lacewing Chrysoperla carnea is mainly released for aphid control, but also attacks mealybugs. Both species have shown potential to control various economically important species of mealybug pests of greenhouse crops. As these predators may be simultaneously present in a crop, the risk of negative interactions between both predators was evaluated in this laboratory study. Individuals of different life stages of either predator were placed together in petri dish arenas and predation was recorded. Attacks between individuals of both species were frequently observed, with lacewing larvae being the dominant predators in most combinations. When mealybug nymphs or lepidopteran eggs were added to the arena, the incidence of attacks between the predators was greatly diminished. The relevance of these observations for the use of the predators in the biological control of greenhouse pests is discussed. Citation: Golsteyn, L.; Mertens, H.; Audenaert, J.; Verhoeven, R.; Gobin, Abstract: The ladybird Cryptolaemus montrouzieri and the green lacewing Chrysoperla carnea have B.; De Clercq, P. -

Endemics Versus Newcomers: the Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria
insects Article Endemics Versus Newcomers: The Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria Jerzy Romanowski 1,* , Piotr Ceryngier 1 , Jaroslav Vetrovec˘ 2, Marta Piotrowska 3 and Karol Szawaryn 4 1 Institute of Biological Sciences, Cardinal Stefan Wyszy´nskiUniversity, Wóycickiego 1/3, 01-938 Warsaw, Poland; [email protected] 2 Buzulucká, 1105 Hradec Králové, Czech Republic; [email protected] 3 Faculty of Biology and Environmental Sciences UKSW, ul. Wóycickiego 1/3, PL-01-938 Warsaw, Poland; [email protected] 4 Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, 00-679 Warsaw, Poland; [email protected] * Correspondence: [email protected] Received: 18 August 2020; Accepted: 14 September 2020; Published: 18 September 2020 Simple Summary: Many plants and animals that live in the Canary Islands belong to the so-called endemic species, i.e., they do not occur outside of this particular region. Several other species have a slightly wider geographical distribution, apart from the Canaries, which also includes some islands of the nearby archipelagos, such as Madeira or the Azores, or the northwestern periphery of Africa. Here, we call such species subendemics. However, the Canary Islands have recently been colonized by a substantial number of immigrants from more or less remote areas. In this paper, based on our field survey and previously published data, we analyzed the fauna of the ladybird beetles (Coccinellidae) of Gran Canaria, one of the central islands of the archipelago. Among 42 ladybird beetle species so far recorded on this island, 17 (40%) are endemics and subendemics, and 21 (50%) probably arrived in Gran Canaria relatively recently, i.e., in the 20th and 21st century. -

VOLUME 2 Polyphaga: Scarabaeoidea Through
VOLUME 2 AMERICAN BEETLES Polyphaga: Scarabaeoidea through Curculionoidea VOLUME 2 AMERICAN BEETLES Polyphaga: Scarabaeoidea through Curculionoidea Edited by the late Ross H. Arnett, Jr., Ph.D. Michael C. Thomas, Ph.D. Paul E. Skelley, Ph.D. and J. Howard Frank, D. Phil. CRC Press Boca Raton London New York Washington, D.C. COVER FIGURES: Center - Coccinellidae, Harmonia axyridus (Palles) [Photo by Fred J. Santana]. Outer rim, clockwise from top: Ripiphoridae, Macrosiagon cruentum (Germar) [by Fred J. Santana]; Meloidae, Lytta magister Horn [by Charles L. Bellamy]; Carabidae, Rhadine exilis (Barr and Lawrence) [by James C. Cokendolpher]; Melyridae, Malachius mirandus (LeConte) [by Max E. Badgley]; Lampyridae, Microphotus angustus LeConte [by Arthur V. Evans]. Library of Congress Cataloging-in-Publication Data American beetles / edited by Ross H. Arnett and Michael C. Thomas. p. cm. Contents: v. 1. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. Includes bibliographical references (p.). ISBN 0-8493-0954-9 (alk. paper : v. 2)) 1. Beetles—North America. I. Arnett, Ross H. II. Thomas, M. C. (Michael Charles). 1948– QL581 .A43 2002 595.76¢097—dc21 00-050809 CIP This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the authors and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher. -

New Record of Predatory Ladybird Beetle (Coleoptera, Coccinellidae) Feeding on Extrafloral Nectaries
SHORT COMMUNICATION New record of predatory ladybird beetle (Coleoptera, Coccinellidae) feeding on extrafloral nectaries Lúcia M. Almeida1, Geovan H. Corrêa1, José A. Giorgi2 & Paschoal C. Grossi1 1Laboratório de Sistemática e Bioecologia de Coleoptera (Insecta), Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19020, 81581–980 Curitiba-PR, Brazil. [email protected]; [email protected]; [email protected] 2Entomologia, Universidade Federal Rural de Pernambuco, Rua Dom Manoel de Medeiros, s/n, Dois Irmãos, 52171–900 Recife-PE, Brazil. [email protected] ABSTRACT. New record of predatory ladybird beetle (Coleoptera, Coccinellidae) feeding on extrafloral nectaries. Feeding by Exoplectra miniata (Germar) on extrafloral nectaries of Inga edulis Mart. was observed in Nova Friburgo, Rio de Janeiro, Brazil. This is the first record of this behavior for Exoplectrini. KEYWORDS. Alimentary resources; Exoplectra miniata; Exoplectrini; Inga edulis; Leguminosae. RESUMO. Novo registro de um coccinelídeo predador (Coleoptera, Coccinellidae) alimentando-se de nectário extrafloral. A ali- mentação de Exoplectra miniata (Germar) em nectários extraflorais de Inga edulis Mart. foi observada em Nova Friburgo, Rio de Janeiro, Brasil. Este é o primeiro registro deste comportamento para Exoplectrini. PALAVRAS-CHAVE. Exoplectra miniata; Exoplectrini; Inga edulis; Leguminosae; recurso alimentar. Coccinellidae is a well-known beetle family, worldwide of feeding strategies for predator coccinellids. Entomophagous distributed -

1 the RESTRUCTURING of ARTHROPOD TROPHIC RELATIONSHIPS in RESPONSE to PLANT INVASION by Adam B. Mitchell a Dissertation Submitt
THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell 1 A dissertation submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Entomology and Wildlife Ecology Winter 2019 © Adam B. Mitchell All Rights Reserved THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell Approved: ______________________________________________________ Jacob L. Bowman, Ph.D. Chair of the Department of Entomology and Wildlife Ecology Approved: ______________________________________________________ Mark W. Rieger, Ph.D. Dean of the College of Agriculture and Natural Resources Approved: ______________________________________________________ Douglas J. Doren, Ph.D. Interim Vice Provost for Graduate and Professional Education I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Douglas W. Tallamy, Ph.D. Professor in charge of dissertation I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Charles R. Bartlett, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Jeffery J. Buler, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. -

Coleoptera: Coccinellidae) As a Host of the Ectoparasitic Fungus Hesperomyces Coccinelloides (Ascomycota: Laboulbeniales: Laboulbeniaceae) in Poland
P O L I S H J O U R N A L OF ENTOMOLOG Y POLSKIE PISMO ENTOMOLOGICZNE VOL. 82: 13-18 Gdańsk 31 March 2013 DOI: 10.2478/v10200-012-0018-7 Stethorus pusillus (Coleoptera: Coccinellidae) as a host of the ectoparasitic fungus Hesperomyces coccinelloides (Ascomycota: Laboulbeniales: Laboulbeniaceae) in Poland PIOTR CERYNGIER Faculty of Biology and Environmental Sciences, Cardinal Stefan Wyszyński University, Wóycickiego 1/3, 01-938 Warsaw, Poland, e-mail: [email protected] ABSTRACT. The laboulbenialean fungus Hesperomyces coccinelloides (THAXTER) THAXTER is known to be an obligate ectoparasite of beetles belonging to the coccinellid subfamily Scymninae. It has been reported from several regions in the world, mostly tropical and subtropical localities. This paper reports the first records of H. coccinelloides from Poland. The parasitic thalli were found growing on the elytra of Stethorus pusillus (HERBST) specimens collected in two adjacent localities in the vicinity of Warsaw. KEY WORDS: Coccinellidae, Stethorus pusillus, Laboulbeniales, Hesperomyces coccinelloides, Poland. INTRODUCTION The order Laboulbeniales consists of about 2000 described species. All of them are obligate ectoparasites of insects and some other arthropods, with beetles (Coleoptera) being their most frequent hosts (WEIR & HAMMOND 1997, MAJEWSKI 2008). Laboulbenialean fungi grow as multicellular thalli on the integument of living arthropods, doing little harm to their hosts (TAVARES 1979, KAUR & MUKERJI 2006). Members of the family Coccinellidae have been reported to host four species of the laboulbenialean genus Hesperomyces THAXTER: H. chilomenis (THAXTER) THAXTER, H. hyperaspidis THAXTER, H. virescens THAXTER and H. coccinelloides (THAXTER) THAXTER. While the former two species are only known from single type specimens and THAXTER’s original (1918, 1931) descriptions, more data are available for H. -

Cryptolaemus Montrouzieri (Mulsant) (Coccinellidae: Scymninae): a Review of Biology, Ecology, and Use in Biological Control With
CAB Reviews 2013 8, No. 005 Review Cryptolaemus montrouzieri (Mulsant) (Coccinellidae: Scymninae): a review of biology, ecology, and use in biological control with particular reference to potential impact on non-target organisms Moses T.K. Kairol*, Oulimathe Paraiso2, Ram Das Gautam3 and Dorothy D. Peterkin4 Address: 1 School of Agricultural and Natural Sciences, University of Maryland, Eastern Shore, Suite 3055, Richard A. Hazel Hall, Princess Anne, MD 21853, USA. 2 Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, FL, USA. 3 Indian Agricultural Research Institute, New Delhi, India. 4 CABI, Gordon Street, Curepe, Trinidad and Tobago, West Indies. *Correspondence: Moses Kairo. E-mail: [email protected] Received: 6 July 2012 Accepted: 19 November 2012 doi: 10.1079/PAVSNNR20138005 The electronic version of this article is the definitive one. It is located here: http://www.cabi.org/cabreviews g CAB International 2013 (Online ISSN 1749-8848) Abstract Over the years, Cryptolaemus montrouzieri Mulsant (Coleoptera: Coccinellidae) has been used in both classical and augmentative biological control programmes. The ladybird is also considered important in certain conservation biological control programmes. This paper provides a critical review of the literature pertaining to its biology, ecology and use, with a particular emphasis on potential impact on non-target organisms. C. montrouzieri has many of the attributes of an effective natural enemy, including a rapid development rate, high reproductive potential, good adaptation to a range of tropical and subtropical climates, high prey consumption rates by both adults and larvae and ease of rearing. The coccinellid has been introduced into at least 64 countries/territories to control more than 16 pest species. -

Coccinellids Associated with the Cotton Aphid (Homoptera: Aphididae) in Northeast Arkansas Cotton'
Coccinellids Associated with the Cotton Aphid (Homoptera: Aphididae) in Northeast Arkansas Cotton' Hugh E. Conway 2 and Timothy J. Kring3 USDA-APHIS-PPQ-CPHST-PDDML Pest Detection, Diagnostic & Management Laboratory, Edinburg, Texas 78541 USA J. Entomol. Sci. 45(2): 129-139 (April 2010) Abstract Adult and larval coccinellids are important predators of the cotton aphid, Aphis gos- sypii Glover, in cotton, Gossypium hirsutum L. Adult and larval Coccinellinae and Scymninae were sampled by beat-pan method and identified to species in a 3-year field study (1999-2001) conducted in northeast Arkansas. The coccinellids observed in descending order of abundance were Hippodarnia convergens Guerin, Scymninae (Scymnus spp. and Diomus spp.), Coleome- gil/a macu/ata (Degeer), Harmonia axyridis (Pallas), and Coccinella septempunctata L. Popula- tion dynamics and community structure by species for C. maculata, H. axyridis and C. septempunctata were unpredictable within and among years. Based on population densities, timing of colonization, and consistent delayed density-dependent relationship with aphid popula- tions, H. convergens and Scymninae genera (Scymnus spp. and Diomus spp.) were the most important predators of cotton aphids in northeast Arkansas. Key Words coccinellid, Coccinellinae, Scymninae, cotton aphid, Aphis gossypii The diverse beneficial insect fauna found in cotton, Gossypium hirsutum L., is well described (Whitcomb and Bell 1964, van den Bosch and Hagen 1966, Wells et al. 2001). The natural enemies commonly credited with controlling the cotton aphid, Aphis gossypll Glover, are the predaceous arthropods including the coccinellids, chrysopids, and syrphids, the parasitoids (braconids), and the entomogenous fungus, Neozygites fresenll (Nowakowski) Batko (Chambers 1986, Frazer 1988, Rosenheim and Cisneros 1994, Steinkraus et al.