First Case of Parthenogenesis in Cryptolaemus Montrouzieri (Mulsant) (Coccinellidae: Scymninae) Under Laboratory Conditions in Morocco
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Beetles
Ireland Red List No. 1 Water beetles Ireland Red List No. 1: Water beetles G.N. Foster1, B.H. Nelson2 & Á. O Connor3 1 3 Eglinton Terrace, Ayr KA7 1JJ 2 Department of Natural Sciences, National Museums Northern Ireland 3 National Parks & Wildlife Service, Department of Environment, Heritage & Local Government Citation: Foster, G. N., Nelson, B. H. & O Connor, Á. (2009) Ireland Red List No. 1 – Water beetles. National Parks and Wildlife Service, Department of Environment, Heritage and Local Government, Dublin, Ireland. Cover images from top: Dryops similaris (© Roy Anderson); Gyrinus urinator, Hygrotus decoratus, Berosus signaticollis & Platambus maculatus (all © Jonty Denton) Ireland Red List Series Editors: N. Kingston & F. Marnell © National Parks and Wildlife Service 2009 ISSN 2009‐2016 Red list of Irish Water beetles 2009 ____________________________ CONTENTS ACKNOWLEDGEMENTS .................................................................................................................................... 1 EXECUTIVE SUMMARY...................................................................................................................................... 2 INTRODUCTION................................................................................................................................................ 3 NOMENCLATURE AND THE IRISH CHECKLIST................................................................................................ 3 COVERAGE ....................................................................................................................................................... -

Cold Tolerance of the Predatory Ladybird Cryptolaemus Montrouzieri
BioControl (2015) 60:199–207 DOI 10.1007/s10526-014-9630-7 Cold tolerance of the predatory ladybird Cryptolaemus montrouzieri Sara Maes • Jean-Claude Gre´goire • Patrick De Clercq Received: 21 May 2014 / Accepted: 15 October 2014 / Published online: 29 October 2014 Ó International Organization for Biological Control (IOBC) 2014 Abstract The effect of low temperature acclimation throughout development and in the first week of their and diet on the supercooling point (SCP, the temperature adult life. Also food source had a significant effect on the at which the insect’s body fluids freeze) and lethal time freezing temperature of C. montrouzieri:theSCPof (LTime, time required to kill 50 % of the population at a ladybirds fed the citrus mealybug, Planococcus citri temperature of 5 °C) of the mealybug destroyer, (Risso)(Hemiptera: Pseudococcidae), was 1.6 °C Cryptolaemus montrouzieri Mulsant (Coleoptera: Coc- higher than the value of -17.2 °C observed for cinellidae), was assessed in the laboratory. The SCP of ladybirds provided with eggs of the flour moth Ephestia acclimated adult ladybirds which were allowed to kuehniella Zeller (Lepidoptera: Pyralidae). However, complete development to adulthood at 18 °Canda neither cold acclimation nor diet had a significant effect 8:16(L:D)h photoperiod, or at 25 °C and a 16:8(L:D)h on the lethal times of C. montrouzieri. Overall, the time photoperiod, and which were subsequently kept at required to kill 50 % of the population at a temperature 10 °C and a 12:12(L:D)h photoperiod for seven days, of 5 °C ranged from 12.8 days for ladybirds fed P. -

Historical, Landscape and Resource Influences on the Coccinellid Community in Missouri
HISTORICAL, LANDSCAPE AND RESOURCE INFLUENCES ON THE COCCINELLID COMMUNITY IN MISSOURI _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the requirements for the Degree Doctor of Philosophy _____________________________________________________ by LAUREN M. DIEPENBROCK Dr. Deborah Finke, Dissertation Supervisor MAY 2014 The undersigned, appointed by the Dean of the Graduate School, have examined the dissertation entitled: HISTORICAL, LANDSCAPE AND RESOURCE INFLUENCES ON THE COCCINELLID COMMUNITY IN MISSOURI Presented by Lauren M. Diepenbrock a candidate for the degree of Doctor of Philosophy and hereby certify that in their opinion is worthy of acceptance ________________________________________________ Dr. Deborah Finke, Dissertation Supervisor, Division of Plant Sciences ________________________________________________ Dr. Richard Houseman, Division of Plant Sciences ________________________________________________ Dr. Bruce Barrett, Division of Plant Sciences ________________________________________________ Dr. John Faaborg, Division of Biological Sciences ACKNOWLEDGEMENTS I would like to thank my Ph. D. advisor, Dr. Deborah Finke for the opportunity to pursue a doctoral degree in insect ecology and for her guidance and support throughout my time at the University of Missouri. I would also like to thank my graduate committee, Drs. Houseman, Barrett and Faaborg for their helpful advice during this academic journey. In addition to my graduate committee, I am grateful for the advice and opportunities that were made available to me by Dr. Rose-Marie Muzika, who introduced me to the Conservation Biology certificate program and all of the great researchers across the university who share my interests in biodiversity conservation. I will always be grateful to Dr. Jeanne Mihail for introducing me to Dr. -

Metacommunities and Biodiversity Patterns in Mediterranean Temporary Ponds: the Role of Pond Size, Network Connectivity and Dispersal Mode
METACOMMUNITIES AND BIODIVERSITY PATTERNS IN MEDITERRANEAN TEMPORARY PONDS: THE ROLE OF POND SIZE, NETWORK CONNECTIVITY AND DISPERSAL MODE Irene Tornero Pinilla Per citar o enllaçar aquest document: Para citar o enlazar este documento: Use this url to cite or link to this publication: http://www.tdx.cat/handle/10803/670096 http://creativecommons.org/licenses/by-nc/4.0/deed.ca Aquesta obra està subjecta a una llicència Creative Commons Reconeixement- NoComercial Esta obra está bajo una licencia Creative Commons Reconocimiento-NoComercial This work is licensed under a Creative Commons Attribution-NonCommercial licence DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode Irene Tornero Pinilla 2020 DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode IRENE TORNERO PINILLA 2020 DOCTORAL PROGRAMME IN WATER SCIENCE AND TECHNOLOGY SUPERVISED BY DR DANI BOIX MASAFRET DR STÉPHANIE GASCÓN GARCIA Thesis submitted in fulfilment of the requirements to obtain the Degree of Doctor at the University of Girona Dr Dani Boix Masafret and Dr Stéphanie Gascón Garcia, from the University of Girona, DECLARE: That the thesis entitled Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode submitted by Irene Tornero Pinilla to obtain a doctoral degree has been completed under our supervision. In witness thereof, we hereby sign this document. Dr Dani Boix Masafret Dr Stéphanie Gascón Garcia Girona, 22nd November 2019 A mi familia Caminante, son tus huellas el camino y nada más; Caminante, no hay camino, se hace camino al andar. -

Triploidy in Chinese Parthenogenetic
COMPARATIVE A peer-reviewed open-access journal CompCytogen 14(1): 1–10 Triploidy(2020) in Chinese parthenogenetic Helophorus orientalis 1 doi: 10.3897/CompCytogen.v14i1.47656 RESEARCH ARTICLE Cytogenetics http://compcytogen.pensoft.net International Journal of Plant & Animal Cytogenetics, Karyosystematics, and Molecular Systematics Triploidy in Chinese parthenogenetic Helophorus orientalis Motschulsky, 1860, further data on parthenogenetic H. brevipalpis Bedel, 1881 and a brief discussion of parthenogenesis in Hydrophiloidea (Coleoptera) Robert B. Angus1,2, Fenglong Jia1 1 Institute of Entomology, School of Life Sciences, Sun Yat-sen University, Guangzhou, 510275, Guangdong, China 2 Department of Life Sciences (Insects), The Natural History Museum, Cromwell Road, London SW7 5BD, UK Corresponding author: Robert B. Angus ([email protected]); Fenglong Jia ([email protected], [email protected]) Academic editor: C. Nokkala | Received 27 October 2019 | Accepted 18 December 2019 | Published 13 January 2020 http://zoobank.org/10F6E8F2-74B9-4966-A775-EAF199B66029 Citation: Angus RB, Jia F (2020) Triploidy in Chinese parthenogenetic Helophorus orientalis Motschulsky, 1860, further data on parthenogenetic H. brevipalpis Bedel, 1881 and a brief discussion of parthenogenesis in Hydrophiloidea (Coleoptera). Comparative Cytogenetics 14(1): 1–10. https://doi.org/10.3897/CompCytogen.v14i1.47656 Abstract The chromosomes of triploid parthenogenetic Helophorus orientalis Motschulsky, 1860 are described from material from two localities in Heilongjiang, China. 3n = 33. All the chromosomes have clear centromeric C-bands, and in the longest chromosome one replicate appears to be consistently longer than the other two. The chromosomes of additional triploid parthenogenetic H. brevipalpis Bedel, 1881, from Spain and Italy, are described. In one Italian population one of the autosomes is represented by only two replicates and another appears more evenly metacentric than in material from Spain and the other Italian locality. -

Contrasting Ladybird Beetle Responses to Urban Environments Across Two US Regions
sustainability Article Context Matters: Contrasting Ladybird Beetle Responses to Urban Environments across Two US Regions Monika Egerer 1,* ID , Kevin Li 2 and Theresa Wei Ying Ong 3,4 ID 1 Environmental Studies Department, University of California, Santa Cruz, Santa Cruz, CA 95064, USA 2 Department of Plant Sciences, University of Göttingen, Göttingen NI 37077, Germany; [email protected] 3 Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA; [email protected] 4 Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ 08540, USA * Correspondence: [email protected]; Tel.: +1-734-775-8950 Received: 8 April 2018; Accepted: 30 May 2018; Published: 1 June 2018 Abstract: Urban agroecosystems offer an opportunity to investigate the diversity and distribution of organisms that are conserved in city landscapes. This information is not only important for conservation efforts, but also has important implications for sustainable agricultural practices. Associated biodiversity can provide ecosystem services like pollination and pest control, but because organisms may respond differently to the unique environmental filters of specific urban landscapes, it is valuable to compare regions that have different abiotic conditions and urbanization histories. In this study, we compared the abundance and diversity of ladybird beetles within urban gardens in California and Michigan, USA. We asked what species are shared, and what species are unique to urban regions. Moreover, we asked how beetle diversity is influenced by the amount and rate of urbanization surrounding sampled urban gardens. We found that the abundance and diversity of beetles, particularly of unique species, respond in opposite directions to urbanization: ladybirds increased with urbanization in California, but decreased with urbanization in Michigan. -

Buglife Ditches Report Vol1
The ecological status of ditch systems An investigation into the current status of the aquatic invertebrate and plant communities of grazing marsh ditch systems in England and Wales Technical Report Volume 1 Summary of methods and major findings C.M. Drake N.F Stewart M.A. Palmer V.L. Kindemba September 2010 Buglife – The Invertebrate Conservation Trust 1 Little whirlpool ram’s-horn snail ( Anisus vorticulus ) © Roger Key This report should be cited as: Drake, C.M, Stewart, N.F., Palmer, M.A. & Kindemba, V. L. (2010) The ecological status of ditch systems: an investigation into the current status of the aquatic invertebrate and plant communities of grazing marsh ditch systems in England and Wales. Technical Report. Buglife – The Invertebrate Conservation Trust, Peterborough. ISBN: 1-904878-98-8 2 Contents Volume 1 Acknowledgements 5 Executive summary 6 1 Introduction 8 1.1 The national context 8 1.2 Previous relevant studies 8 1.3 The core project 9 1.4 Companion projects 10 2 Overview of methods 12 2.1 Site selection 12 2.2 Survey coverage 14 2.3 Field survey methods 17 2.4 Data storage 17 2.5 Classification and evaluation techniques 19 2.6 Repeat sampling of ditches in Somerset 19 2.7 Investigation of change over time 20 3 Botanical classification of ditches 21 3.1 Methods 21 3.2 Results 22 3.3 Explanatory environmental variables and vegetation characteristics 26 3.4 Comparison with previous ditch vegetation classifications 30 3.5 Affinities with the National Vegetation Classification 32 Botanical classification of ditches: key points -

Biological Pest Controls: Possibilities for Improved Implementation in Iowa David B
Proceedings of the Integrated Crop Management Proceedings of the 1990 Crop Production and Conference Protection Conference Dec 19th, 12:00 AM Biological Pest Controls: Possibilities for Improved Implementation in Iowa David B. Orr Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/icm Part of the Agriculture Commons, and the Entomology Commons Orr, David B., "Biological Pest Controls: Possibilities for Improved Implementation in Iowa" (1990). Proceedings of the Integrated Crop Management Conference. 23. https://lib.dr.iastate.edu/icm/1990/proceedings/23 This Event is brought to you for free and open access by the Conferences and Symposia at Iowa State University Digital Repository. It has been accepted for inclusion in Proceedings of the Integrated Crop Management Conference by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. BIOLOGICAL PEST CONTROLS: POSSIBILITIES FOR IMPROVED IMPLEMENTATION IN IOWA David B. orr Temporary Assistant Professor Department of Entomology Introduction Chemical insecticides are the standard means for reducing populations of insect pests in agricultural and horticultural settings. However, government regulations are restricting the use of a variety of pesticides for many agricultural uses. The requirement of registration for all pesticides labelled before 1984 has already resulted in the cancellation of approximately 20,000 pesticide registrations in 1989. In addition, pest resistance to insecticides (over 400 species), and an increased sensitivity in the general public to possible environmental and health hazards are forcing the agricultural industry to search for alternatives to chemical control. Integrated Pest Management (IPM) offers the possibility of reduced pesticide use, and more sustainable pest management systems. -

Biological Studies and Evaluation of Scymnus Coniferarum Crotch, a Predator of Hemlock Woolly Adelgid from Western North America
Biological studies and evaluation of Scymnus coniferarum Crotch, a predator of hemlock woolly adelgid from western North America Molly Norton Darr Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Entomology Scott M. Salom, Chair Timothy J. Kring Michael E. Montgomery James P. Parkman Paul E. Marek May 1, 2017 Blacksburg, VA Keywords: Biological control, Hemlock woolly adelgid, Adelges tsugae, Scymnus coniferarum, Tsuga heterophylla, Tsuga canadensis Biological studies and evaluation of Scymnus coniferarum Crotch, a predator of hemlock woolly adelgid from western North America Molly Norton Darr ABSTRACT (ACADEMIC) The hemlock woolly adelgid (HWA), Adelges tsugae Annand, is an invasive pest of eastern hemlock, Tsuga canadensis (L.) Carriere and Carolina hemlock Tsuga caroliniana Englem. in the eastern United States. A newly reported beetle predator for HWA, Scymnus (Pullus) coniferarum Crotch (Coleoptera: Cocinellidae) preys on the pest in the western United States, and was approved for release in the eastern United States for the control of HWA. This research investigated the viability of S. coniferarum as a biological control agent of A. tsugae in the eastern United States, as well as the ecological dynamics between S. coniferarum and host prey species in its native range of western North America. In objective one, S. coniferarum predation, reproductive potential, and survival were evaluated in field-cages on adelgid infested T. canadensis in southwestern Virginia. Adult S. coniferarum fed on both generations and all life stages of A. tsugae at rates comparable to other adelgid-specific predators, and survived for extended periods of time in the field. -
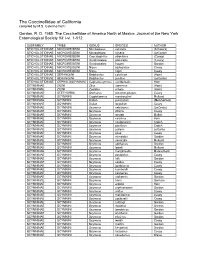
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Review of Clitostethus Weise, Parastethorus Pang Et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/327149552 Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan Article in Oriental insects · August 2018 DOI: 10.1080/00305316.2018.1492987 CITATIONS READS 0 20 4 authors, including: Zafar Iqbal Muhammad Farooq Nasir PMAS - Arid Agriculture University 7 PUBLICATIONS 0 CITATIONS 21 PUBLICATIONS 56 CITATIONS SEE PROFILE SEE PROFILE Imran Bodlah PMAS - Arid Agriculture University 69 PUBLICATIONS 119 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Faunastic study of Stratiomyidae (Diptera) from Pakistan View project termite cibtrol View project All content following this page was uploaded by Imran Bodlah on 28 August 2018. The user has requested enhancement of the downloaded file. Oriental Insects ISSN: 0030-5316 (Print) 2157-8745 (Online) Journal homepage: http://www.tandfonline.com/loi/toin20 Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan Zafar Iqbal, Muhammad Farooq Nasir, Imran Bodlah & Karol Szawaryn To cite this article: Zafar Iqbal, Muhammad Farooq Nasir, Imran Bodlah & Karol Szawaryn (2018): Review of Clitostethus Weise, Parastethorus Pang et Mao and Stethorus Weise (Coleoptera: Coccinellidae) from Pakistan, Oriental Insects, DOI: 10.1080/00305316.2018.1492987 To link to this article: https://doi.org/10.1080/00305316.2018.1492987 Published -

Intraguild Interactions Between the Mealybug Predators Cryptolaemus Montrouzieri and Chrysoperla Carnea
insects Article Intraguild Interactions between the Mealybug Predators Cryptolaemus montrouzieri and Chrysoperla carnea Laura Golsteyn 1, Hana Mertens 1, Joachim Audenaert 2, Ruth Verhoeven 2, Bruno Gobin 2 and Patrick De Clercq 1,* 1 Department of Plants and Crops, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium; [email protected] (L.G.); [email protected] (H.M.) 2 PCS—Ornamental Plant Research, Schaessestraat 18, B-9070 Destelbergen, Belgium; [email protected] (J.A.); [email protected] (R.V.); [email protected] (B.G.) * Correspondence: [email protected]; Tel.: +32-92-646-158 Simple Summary: The ladybird Cryptolaemus montrouzieri is a widely commercialized biological control agent of mealybugs. The green lacewing Chrysoperla carnea is mainly released for aphid control, but also attacks mealybugs. Both species have shown potential to control various economically important species of mealybug pests of greenhouse crops. As these predators may be simultaneously present in a crop, the risk of negative interactions between both predators was evaluated in this laboratory study. Individuals of different life stages of either predator were placed together in petri dish arenas and predation was recorded. Attacks between individuals of both species were frequently observed, with lacewing larvae being the dominant predators in most combinations. When mealybug nymphs or lepidopteran eggs were added to the arena, the incidence of attacks between the predators was greatly diminished. The relevance of these observations for the use of the predators in the biological control of greenhouse pests is discussed. Citation: Golsteyn, L.; Mertens, H.; Audenaert, J.; Verhoeven, R.; Gobin, Abstract: The ladybird Cryptolaemus montrouzieri and the green lacewing Chrysoperla carnea have B.; De Clercq, P.