Optimizing Laboratory Rearing of a Key Pollinator, Bombus Impatiens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification of the Apidae (Hymenoptera)
Utah State University DigitalCommons@USU Mi Bee Lab 9-21-1990 Classification of the Apidae (Hymenoptera) Charles D. Michener University of Kansas Follow this and additional works at: https://digitalcommons.usu.edu/bee_lab_mi Part of the Entomology Commons Recommended Citation Michener, Charles D., "Classification of the Apidae (Hymenoptera)" (1990). Mi. Paper 153. https://digitalcommons.usu.edu/bee_lab_mi/153 This Article is brought to you for free and open access by the Bee Lab at DigitalCommons@USU. It has been accepted for inclusion in Mi by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. 4 WWvyvlrWryrXvW-WvWrW^^ I • • •_ ••^«_«).•>.• •.*.« THE UNIVERSITY OF KANSAS SCIENC5;^ULLETIN LIBRARY Vol. 54, No. 4, pp. 75-164 Sept. 21,1990 OCT 23 1990 HARVARD Classification of the Apidae^ (Hymenoptera) BY Charles D. Michener'^ Appendix: Trigona genalis Friese, a Hitherto Unplaced New Guinea Species BY Charles D. Michener and Shoichi F. Sakagami'^ CONTENTS Abstract 76 Introduction 76 Terminology and Materials 77 Analysis of Relationships among Apid Subfamilies 79 Key to the Subfamilies of Apidae 84 Subfamily Meliponinae 84 Description, 84; Larva, 85; Nest, 85; Social Behavior, 85; Distribution, 85 Relationships among Meliponine Genera 85 History, 85; Analysis, 86; Biogeography, 96; Behavior, 97; Labial palpi, 99; Wing venation, 99; Male genitalia, 102; Poison glands, 103; Chromosome numbers, 103; Convergence, 104; Classificatory questions, 104 Fossil Meliponinae 105 Meliponorytes, -

Anthidium Manicatum, an Invasive Bee, Excludes a Native Bumble Bee, Bombus Impatiens, from floral Resources
Biol Invasions https://doi.org/10.1007/s10530-018-1889-7 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL PAPER Anthidium manicatum, an invasive bee, excludes a native bumble bee, Bombus impatiens, from floral resources Kelsey K. Graham . Katherine Eaton . Isabel Obrien . Philip T. Starks Received: 15 April 2018 / Accepted: 21 November 2018 Ó Springer Nature Switzerland AG 2018 Abstract Anthidium manicatum is an invasive pol- response to A. manicatum presence. We found that B. linator reaching widespread distribution in North impatiens avoided foraging near A. manicatum in both America. Male A. manicatum aggressively defend years; but despite this resource exclusion, we found no floral territories, attacking heterospecific pollinators. evidence of fitness consequences for B. impatiens. Female A. manicatum are generalists, visiting many of These results suggest A. manicatum pose as significant the same plants as native pollinators. Because of A. resource competitors, but that B. impatiens are likely manicatum’s rapid range expansion, the territorial able to compensate for this resource loss by finding behavior of males, and the potential for female A. available resources elsewhere. manicatum to be significant resource competitors, invasive A. manicatum have been prioritized as a Keywords Exotic species Á Resource competition Á species of interest for impact assessment. But despite Interspecific competition Á Foraging behavior Á concerns, there have been no empirical studies inves- Pollination tigating the impact of A. manicatum on North Amer- ican pollinators. Therefore, across a two-year study, we monitored foraging behavior and fitness of the common eastern bumble bee (Bombus impatiens) in Introduction With increasing movement of goods and people Electronic supplementary material The online version of around the world, introduction of exotic species is this article (https://doi.org/10.1007/s10530-018-1889-7) con- increasing at an unprecedented rate (Ricciardi et al. -
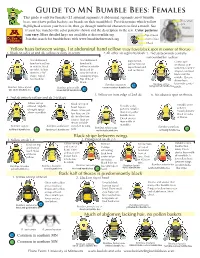
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

Beewalk Report 2020
BeeWalk Annual Report 2020 Richard Comont and Helen Dickinson BeeWalk Annual Report 2020 About BeeWalk BeeWalk is a standardised bumblebee-monitoring scheme active across Great Britain since 2008, and this report covers the period 2008–19. The scheme protocol involves volunteer BeeWalkers walking the same fixed route (a transect) at least once a month between March and October (inclusive). This covers the full flight period of the bumblebees, including emergence from overwintering and workers tailing off. Volunteers record the abundance of each bumblebee species seen in a 4 m x 4 m x 2 m ‘recording box’ in order to standardise between habitats and observers. It is run by Dr Richard Comont and Helen Dickinson of the Bumblebee Conservation Trust (BBCT). To contact the scheme organisers, please email [email protected]. Acknowledgements We are indebted to the volunteers and organisations past and present who have contributed data to the scheme or have helped recruit or train others in connection with it. Thanks must also go to all the individuals and organisations who allow or even actively promote access to their land for bumblebee recording. We would like to thank the financial contribution by the Redwing Trust, Esmée Fairbairn Foundation, Garfield Weston Foundation and the many other organisations, charitable trusts and individuals who have supported the BeeWalk scheme in particular, and the Bumblebee Conservation Trust in general. In particular, the Biological Records Centre have provided website support, data storage and desk space free of charge. Finally, we would like to thank the photographers who have allowed their excellent images to be used as part of this BeeWalk Annual Report. -

Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture
Matteson and Langellotto: URBAN BUMBLE BEE ABUNDANCE Cities and the Environment 2009 Volume 2, Issue 1 Article 5 Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture Kevin C. Matteson and Gail A. Langellotto Abstract A variety of crops are grown in New York City community gardens. Although the production of many crops benefits from pollination by bees, little is known about bee abundance in urban community gardens or which crops are specifically dependent on bee pollination. In 2005, we compiled a list of crop plants grown within 19 community gardens in New York City and classified these plants according to their dependence on bee pollination. In addition, using mark-recapture methods, we estimated the abundance of a potentially important pollinator within New York City urban gardens, the common eastern bumble bee (Bombus impatiens). This species is currently recognized as a valuable commercial pollinator of greenhouse crops. However, wild populations of B. impatiens are abundant throughout its range, including in New York City community gardens, where it is the most abundant native bee species present and where it has been observed visiting a variety of crop flowers. We conservatively counted 25 species of crop plants in 19 surveyed gardens. The literature suggests that 92% of these crops are dependent, to some degree, on bee pollination in order to set fruit or seed. Bombus impatiens workers were observed visiting flowers of 78% of these pollination-dependent crops. Estimates of the number of B. impatiens workers visiting individual gardens during the study period ranged from 3 to 15 bees per 100 m2 of total garden area and 6 to 29 bees per 100 m2 of garden floral area. -

Phylogenetic Analysis of the Corbiculate Bee Tribes Based on 12 Nuclear Protein-Coding Genes (Hymenoptera: Apoidea: Apidae) Atsushi Kawakita, John S
Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae) Atsushi Kawakita, John S. Ascher, Teiji Sota, Makoto Kato, David W. Roubik To cite this version: Atsushi Kawakita, John S. Ascher, Teiji Sota, Makoto Kato, David W. Roubik. Phylogenetic anal- ysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae). Apidologie, Springer Verlag, 2008, 39 (1), pp.163-175. hal-00891935 HAL Id: hal-00891935 https://hal.archives-ouvertes.fr/hal-00891935 Submitted on 1 Jan 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie 39 (2008) 163–175 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2007046 Original article Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae)* Atsushi Kawakita1, John S. Ascher2, Teiji Sota3,MakotoKato 1, David W. Roubik4 1 Graduate School of Human and Environmental Studies, Kyoto University, Kyoto, Japan 2 Division of Invertebrate Zoology, American Museum of Natural History, New York, USA 3 Department of Zoology, Graduate School of Science, Kyoto University, Kyoto, Japan 4 Smithsonian Tropical Research Institute, Balboa, Ancon, Panama Received 2 July 2007 – Revised 3 October 2007 – Accepted 3 October 2007 Abstract – The corbiculate bees comprise four tribes, the advanced eusocial Apini and Meliponini, the primitively eusocial Bombini, and the solitary or communal Euglossini. -

Bumblebee in the UK
There are 24 species of bumblebee in the UK. This field guide contains illustrations and descriptions of the eight most common species. All illustrations 1.5x actual size. There has been a marked decline in the diversity and abundance of wild bees across Europe in recent decades. In the UK, two species of bumblebee have become extinct within the last 80 years, and seven species are listed in the Government’s Biodiversity Action Plan as priorities for conservation. This decline has been largely attributed to habitat destruction and fragmentation, as a result of Queen Worker Male urbanisation and the intensification of agricultural practices. Common The Centre for Agroecology and Food Security is conducting Tree bumblebee (Bombus hypnorum) research to encourage and support bumblebees in food Bumblebees growing areas on allotments and in gardens. Bees are of the United Kingdom Queens, workers and males all have a brown-ginger essential for food security, and are regarded as the most thorax, and a black abdomen with a white tail. This important insect pollinators worldwide. Of the 100 crop species that provide 90% of the world’s food, over 70 are recent arrival from France is now present across most pollinated by bees. of England and Wales, and is thought to be moving northwards. Size: queen 18mm, worker 14mm, male 16mm The Centre for Agroecology and Food Security (CAFS) is a joint initiative between Coventry University and Garden Organic, which brings together social and natural scientists whose collective research expertise in the fields of agriculture and food spans several decades. The Centre conducts critical, rigorous and relevant research which contributes to the development of agricultural and food production practices which are economically sound, socially just and promote long-term protection of natural Queen Worker Male resources. -

Conserving Missouri's Wild and Managed Pollinators
Conserving Missouri’s Wild and Managed Pollinators At the heart of the pollination issue lies our bounty of foods such as peaches, Go to the bee, thou poet: strawberries, squash and apples. These and other foods requiring pollination have consider her ways and be wise. been staples in the human diet for centuries, and their pollinators have been highly — George Bernard Shaw revered since ancient times. From Egyptian hieroglyphics and Native American cave paintings to Greek mythology and English poetry, bees and butterflies have been a source of fascination and awe for millennia (Figure 1). Yet, over the past century, pollinator numbers have suffered declines. During this time, global development, a booming human population, and industrial agriculture brought about drastic landscape changes. These changes have resulted in greater losses of forage and nesting resources for pollinators than ever before seen. The ecosystem service of pollination, once taken for granted, is now potentially threatened as many pollinator species face declines in Missouri, the United States and many regions around the world. Pollinators are critically important for natural ecosystems and crop production. Through pollination, they perform roles essential to human welfare. Heightened public awareness of their services and of their declines over the past two decades has prompted action, but much remains to be done. This publication introduces issues regarding the conservation of pollinators in Missouri. It explores why pollinators are crucial, what major threats confront them, what conservation steps are being taken, and how you can help. It highlights bees over other pollinators as bees are the most important for both agricultural and natural pollination in Missouri. -

Individual Versus Collective Cognition in Social Insects
Individual versus collective cognition in social insects Ofer Feinermanᴥ, Amos Kormanˠ ᴥ Department of Physics of Complex Systems, Weizmann Institute of Science, 7610001, Rehovot, Israel. Email: [email protected] ˠ Institut de Recherche en Informatique Fondamentale (IRIF), CNRS and University Paris Diderot, 75013, Paris, France. Email: [email protected] Abstract The concerted responses of eusocial insects to environmental stimuli are often referred to as collective cognition on the level of the colony.To achieve collective cognitiona group can draw on two different sources: individual cognitionand the connectivity between individuals.Computation in neural-networks, for example,is attributedmore tosophisticated communication schemes than to the complexity of individual neurons. The case of social insects, however, can be expected to differ. This is since individual insects are cognitively capable units that are often able to process information that is directly relevant at the level of the colony.Furthermore, involved communication patterns seem difficult to implement in a group of insects since these lack clear network structure.This review discusses links between the cognition of an individual insect and that of the colony. We provide examples for collective cognition whose sources span the full spectrum between amplification of individual insect cognition and emergent group-level processes. Introduction The individuals that make up a social insect colony are so tightly knit that they are often regarded as a single super-organism(Wilson and Hölldobler, 2009). This point of view seems to go far beyond a simple metaphor(Gillooly et al., 2010)and encompasses aspects of the colony that are analogous to cell differentiation(Emerson, 1939), metabolic rates(Hou et al., 2010; Waters et al., 2010), nutrient regulation(Behmer, 2009),thermoregulation(Jones, 2004; Starks et al., 2000), gas exchange(King et al., 2015), and more. -

Bombus Terrestris) Colonies
veterinary sciences Article Replicative Deformed Wing Virus Found in the Head of Adults from Symptomatic Commercial Bumblebee (Bombus terrestris) Colonies Giovanni Cilia , Laura Zavatta, Rosa Ranalli, Antonio Nanetti * and Laura Bortolotti CREA Research Centre for Agriculture and Environment, Via di Saliceto 80, 40128 Bologna, Italy; [email protected] (G.C.); [email protected] (L.Z.); [email protected] (R.R.); [email protected] (L.B.) * Correspondence: [email protected] Abstract: The deformed wing virus (DWV) is one of the most common honey bee pathogens. The virus may also be detected in other insect species, including Bombus terrestris adults from wild and managed colonies. In this study, individuals of all stages, castes, and sexes were sampled from three commercial colonies exhibiting the presence of deformed workers and analysed for the presence of DWV. Adults (deformed individuals, gynes, workers, males) had their head exscinded from the rest of the body and the two parts were analysed separately by RT-PCR. Juvenile stages (pupae, larvae, and eggs) were analysed undissected. All individuals tested positive for replicative DWV, but deformed adults showed a higher number of copies compared to asymptomatic individuals. Moreover, they showed viral infection in their heads. Sequence analysis indicated that the obtained DWV amplicons belonged to a strain isolated in the United Kingdom. Further studies are needed to Citation: Cilia, G.; Zavatta, L.; characterize the specific DWV target organs in the bumblebees. The result of this study indicates the Ranalli, R.; Nanetti, A.; Bortolotti, L. evidence of DWV infection in B. -

Hoverflies: the Garden Mimics
Article Hoverflies: the garden mimics. Edmunds, Malcolm Available at http://clok.uclan.ac.uk/1620/ Edmunds, Malcolm (2008) Hoverflies: the garden mimics. Biologist, 55 (4). pp. 202-207. ISSN 0006-3347 It is advisable to refer to the publisher’s version if you intend to cite from the work. For more information about UCLan’s research in this area go to http://www.uclan.ac.uk/researchgroups/ and search for <name of research Group>. For information about Research generally at UCLan please go to http://www.uclan.ac.uk/research/ All outputs in CLoK are protected by Intellectual Property Rights law, including Copyright law. Copyright, IPR and Moral Rights for the works on this site are retained by the individual authors and/or other copyright owners. Terms and conditions for use of this material are defined in the policies page. CLoK Central Lancashire online Knowledge www.clok.uclan.ac.uk Hoverflies: the garden mimics Mimicry offers protection from predators by convincing them that their target is not a juicy morsel after all. it happens in our backgardens too and the hoverfly is an expert at it. Malcolm overflies are probably the best the mimic for the model and do not attack Edmunds known members of tbe insect or- it (Edmunds, 1974). Mimicry is far more Hder Diptera after houseflies, blue widespread in the tropics than in temperate bottles and mosquitoes, but unlike these lands, but we have some of the most superb insects they are almost universally liked examples of mimicry in Britain, among the by the general public. They are popular hoverflies. -

Andy Clark and His Critics
Andy Clark and His Critics E D I T E D BY MATTEO COLOMBO ELIZABETH IRVINE and MOG STAPLETON 1 1 Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford is a registered trade mark of Oxford University Press in the UK and certain other countries. Published in the United States of America by Oxford University Press 198 Madison Avenue, New York, NY 10016, United States of America. © Oxford University Press 2019 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, by license, or under terms agreed with the appropriate reproduction rights organization. Inquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above. You must not circulate this work in any other form and you must impose this same condition on any acquirer. CIP data is on file at the Library of Congress ISBN 978– 0– 19– 066281– 3 9 8 7 6 5 4 3 2 1 Printed by Sheridan Books, Inc., United States of America CONTENTS Foreword ix Daniel C. Dennett Acknowledgements xi List of Contributors xiii Introduction 1 Matteo Colombo, Liz Irvine, and Mog Stapleton PART 1 EXTENSIONS AND ALTERATIONS 1. Extended Cognition and Extended Consciousness 9 David J. Chalmers 2. The Elusive Extended Mind: Extended Information Processing Doesn’t Equal Extended Mind 21 Fred Adams 3.