Online Atmospheric Pressure Chemical Ionization Ion Trap Mass Spectrometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ion Trap Quantum Computer Selected Topics
Ion Trap Quantum Computer Selected Topics Ruben Andrist & Thomas Uehlinger 1 Outline Ion Traps Part I Deutsch-Josza Deutsch-Josza algorithm Problem Algorithm Implementation Part II Scalability Scalability of ion trap Problem Move ions quantum computers Microtraps Photons Charges Part III Roundup Roundup & Outlook Ruben Andrist Thomas Uehlinger 2 0:98.')4Part+8, 6: ,I39:2 .07.:09 $ " % H " !1 1# %1#% Quantum Algorithms H !1 1# %! 1#% 0"0.9743(.6:-(9 ( 5 ! ! 5 &1 ( 5 " "$1&!' 5 249(43,"6:-(9 # H !1 1# %1#%!4389,39( H !1 1# %! 1#% !,$,3.0/) ":,39:2,3,()8+8 ,+;08 9/0 7+,/9,38107 ,1907 ,8+3,(0 20,8:702039 3 W 0:98.)*# ,48.,*!74. # $4. 343/439*;99> W A0B803*C ):,3E*",3/"C*,2-7A/E0; > Implementation of the Deutsch-Josza Ion Traps algorithm using trapped ions Deutsch-Josza Problem ! show suitability of ion traps Algorithm Implementation ! relatively simple algorithm Scalability Problem ! using only one trapped ion Move ions Microtraps Photons Charges Paper: Nature 421, 48-50 (2 January 2003), doi:10.1038/nature01336; Institut für Experimentalphysik, Universität Innsbruck ! Roundup ! MIT Media Laboratory, Cambridge, Massachusetts Ruben Andrist Thomas Uehlinger 4 The two problems Ion Traps The Deutsch-Josza Problem: Deutsch-Josza ! Bob uses constant or balanced function Problem Alice has to determine which kind Algorithm ! Implementation Scalability Problem The Deutsch Problem: Move ions Bob uses a fair or forged coin Microtraps ! Photons ! Alice has to determine which kind Charges Roundup Ruben Andrist Thomas Uehlinger 5 Four -

240 Ion Trap GC/MS External Ionization User's Guide
Agilent 240 Ion Trap GC/MS External Ionization User’s Guide Agilent Technologies Notices © Agilent Technologies, Inc. 2011 Warranty (June 1987) or DFAR 252.227-7015 (b)(2) (November 1995), as applicable in any No part of this manual may be reproduced The material contained in this docu- technical data. in any form or by any means (including ment is provided “as is,” and is sub- electronic storage and retrieval or transla- ject to being changed, without notice, tion into a foreign language) without prior Safety Notices agreement and written consent from in future editions. Further, to the max- Agilent Technologies, Inc. as governed by imum extent permitted by applicable United States and international copyright law, Agilent disclaims all warranties, CAUTION either express or implied, with regard laws. to this manual and any information A CAUTION notice denotes a Manual Part Number contained herein, including but not hazard. It calls attention to an oper- limited to the implied warranties of ating procedure, practice, or the G3931-90003 merchantability and fitness for a par- like that, if not correctly performed ticular purpose. Agilent shall not be or adhered to, could result in Edition liable for errors or for incidental or damage to the product or loss of First edition, May 2011 consequential damages in connection important data. Do not proceed with the furnishing, use, or perfor- beyond a CAUTION notice until the Printed in USA mance of this document or of any indicated conditions are fully Agilent Technologies, Inc. information contained herein. Should understood and met. 5301 Stevens Creek Boulevard Agilent and the user have a separate Santa Clara, CA 95051 USA written agreement with warranty terms covering the material in this document that conflict with these WARNING terms, the warranty terms in the sep- A WARNING notice denotes a arate agreement shall control. -

Contents • Introduction to Proteomics and Mass Spectrometry
Contents • Introduction to Proteomics and Mass spectrometry - What we need to know in our quest to explain life - Fundamental things you need to know about mass spectrometry • Interfaces and ion sources - Electrospray ionization (ESI) - conventional and nanospray - Heated nebulizer atmospheric pressure chemical ionization - Matrix assisted laser desorption • Types of MS analyzers - Magnetic sector - Quadrupole - Time-of-flight - Hybrid - Ion trap In the next step in our quest to explain what is life The human genome project has largely been completed and many other genomes are surrendering to the gene sequencers. However, all this knowledge does not give us the information that is needed to explain how living cells work. To do that, we need to study proteins. In 2002, mass spectrometry has developed to the point where it has the capacity to obtain the "exact" molecular weight of many macromolecules. At the present time, this includes proteins up to 150,000 Da. Proteins of higher molecular weights (up to 500,000 Da) can also be studied by mass spectrometry, but with less accuracy. The paradigm for sequencing of peptides and identification of proteins has changed – because of the availability of the human genome database, peptides can be identified merely by their masses or by partial sequence information, often in minutes, not hours. This new capacity is shifting the emphasis of biomedical research back to the functional aspects of cell biochemistry, the expression of particular sets of genes and their gene products, the proteins of the cell. These are the new goals of the biological scientist: o to know which proteins are expressed in each cell, preferably one cell at a time o to know how these proteins are modified, information that cannot necessarily be deduced from the nucleotide sequence of individual genes. -
Mass Spectrometry (Technically Not Spectroscopy)
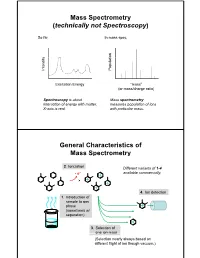
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

An Introduction to Mass Spectrometry
An Introduction to Mass Spectrometry by Scott E. Van Bramer Widener University Department of Chemistry One University Place Chester, PA 19013 [email protected] http://science.widener.edu/~svanbram revised: September 2, 1998 © Copyright 1997 TABLE OF CONTENTS INTRODUCTION ........................................................... 4 SAMPLE INTRODUCTION ....................................................5 Direct Vapor Inlet .......................................................5 Gas Chromatography.....................................................5 Liquid Chromatography...................................................6 Direct Insertion Probe ....................................................6 Direct Ionization of Sample ................................................6 IONIZATION TECHNIQUES...................................................6 Electron Ionization .......................................................7 Chemical Ionization ..................................................... 9 Fast Atom Bombardment and Secondary Ion Mass Spectrometry .................10 Atmospheric Pressure Ionization and Electrospray Ionization ....................11 Matrix Assisted Laser Desorption/Ionization ................................ 13 Other Ionization Methods ................................................13 Self-Test #1 ...........................................................14 MASS ANALYZERS .........................................................14 Quadrupole ............................................................15 -

Orbitrap Fusion Tribrid Mass Spectrometer
MASS SPECTROMETRY Product Specifications Thermo Scientific Orbitrap Fusion Tribrid Mass Spectrometer Unmatched analytical performance, revolutionary MS architecture The Thermo Scientific™ Orbitrap Fusion™ mass spectrometer combines the best of quadrupole, Orbitrap, and linear ion trap mass analysis in a revolutionary Thermo Scientific™ Tribrid™ architecture that delivers unprecedented depth of analysis. It enables life scientists working with even the most challenging samples—samples of low abundance, high complexity, or difficult-to-analyze chemical structure—to identify more compounds faster, quantify them more accurately, and elucidate molecular composition more thoroughly. • Tribrid architecture combines quadrupole, followed by ETD or EThCD for glycopeptide linear ion trap, and Orbitrap mass analyzers characterization or HCD followed by CID • Multiple fragmentation techniques—CID, for small-molecule structural analysis. HCD, and optional ETD and EThCD—are available at any stage of MSn, with The ultrahigh resolution of the Orbitrap mass subsequent mass analysis in either the ion analyzer increases certainty of analytical trap or Orbitrap mass analyzer results, enabling molecular-weight • Parallelization of MS and MSn acquisition determination for intact proteins and confident to maximize the amount of high-quality resolution of isobaric species. The unsurpassed data acquired scan rate and resolution of the system are • Next-generation ion sources and ion especially useful when dealing with complex optics increase system ease of operation and robustness and low-abundance samples in proteomics, • Innovative instrument control software metabolomics, glycomics, lipidomics, and makes setup easier, methods more similar applications. powerful, and operation more intuitive The intuitive user interface of the tune editor The Orbitrap Fusion Tribrid MS can perform and method editor makes instrument calibration a wide variety of analyses, from in-depth and method development easier. -

Methods of Ion Generation
Chem. Rev. 2001, 101, 361−375 361 Methods of Ion Generation Marvin L. Vestal PE Biosystems, Framingham, Massachusetts 01701 Received May 24, 2000 Contents I. Introduction 361 II. Atomic Ions 362 A. Thermal Ionization 362 B. Spark Source 362 C. Plasma Sources 362 D. Glow Discharge 362 E. Inductively Coupled Plasma (ICP) 363 III. Molecular Ions from Volatile Samples. 364 A. Electron Ionization (EI) 364 B. Chemical Ionization (CI) 365 C. Photoionization (PI) 367 D. Field Ionization (FI) 367 IV. Molecular Ions from Nonvolatile Samples 367 Marvin L. Vestal received his B.S. and M.S. degrees, 1958 and 1960, A. Spray Techniques 367 respectively, in Engineering Sciences from Purdue Univesity, Layfayette, IN. In 1975 he received his Ph.D. degree in Chemical Physics from the B. Electrospray 367 University of Utah, Salt Lake City. From 1958 to 1960 he was a Scientist C. Desorption from Surfaces 369 at Johnston Laboratories, Inc., in Layfayette, IN. From 1960 to 1967 he D. High-Energy Particle Impact 369 became Senior Scientist at Johnston Laboratories, Inc., in Baltimore, MD. E. Low-Energy Particle Impact 370 From 1960 to 1962 he was a Graduate Student in the Department of Physics at John Hopkins University. From 1967 to 1970 he was Vice F. Low-Energy Impact with Liquid Surfaces 371 President at Scientific Research Instruments, Corp. in Baltimore, MD. From G. Flow FAB 371 1970 to 1975 he was a Graduate Student and Research Instructor at the H. Laser Ionization−MALDI 371 University of Utah, Salt Lake City. From 1976 to 1981 he became I. -

Selective Chemical Ionization of Nitrogen and Sulfur Heterocycles in Petroleum Fractions by Ion Trap Mass Spectrometry
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Selective Chemical Ionization of Nitrogen and Sulfur Heterocycles in Petroleum Fractions by Ion Trap Mass Spectrometry C. S. Creaser*, F. Krokos, and K. E. O’Neill Shod of Chemical Sciences, University of East Anglia, Norwich, United Kingdom M. J. C. Smith and P. G. McDowell BP Research, Sunbury-on-Thames, Middlesex, United Kingdom A procedure is reported for the selective ammonia chemical ionization of some nitrogen and sulfur heterocycles in petroleum fractions using ion trap mass spectrometry (ITMS). The ion trap scan routine is designed to optimize the population of ammonium reagent ions and eject from the trap (by radio frequency/direct current isolation) electron ionization products formed during reagent ion formation prior to ionization of the sample. The ITMS procedure is compared with standard ion trap detector and conventional quadrupole ammonia chemi- cal ionization for the determination of nitrogen and sulfur heterocycles in gas oil and kerosine samples. Greatly enhanced selectivity is shown for the ITMS procedure by sup res- sion of competing charge-exchange processes. (1 Am Sot Mass Spectrum 1993, 4, 322-326 ‘; he removal of organic compounds containing reagent ion NH;, is formed via the reactions [5] nitrogen and sulfur is an important part of the T petroleum-refining process 111. Their presence in NH, ‘1 NH;’ (1) petroleum distillates results in the formation of envi- NH, f NH:‘+ NH; + NH; (2) ronmental pollutants (SO,, NO,) during combustion, and their reactivity can lead to catalyst poisoning Whereas NH: is unreactive toward a wide range of in refinery processes. -

A Researcher's Guide to Mass Spectrometry‐Based Proteomics
Proteomics 2016, 16, 2435–2443 DOI 10.1002/pmic.201600113 2435 TUTORIAL A researcher’s guide to mass spectrometry-based proteomics John P. Savaryn1,2∗, Timothy K. Toby3∗ and Neil L. Kelleher1,3,4 1 Proteomics Center of Excellence, Northwestern University, Evanston, Illinois, USA 2 Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA 3 Department of Molecular Biosciences, Northwestern University, Evanston, Illinois, USA 4 Department of Chemistry, Northwestern University, Evanston, Illinois, USA Mass spectrometry (MS) is widely recognized as a powerful analytical tool for molecular re- Received: February 24, 2016 search. MS is used by researchers around the globe to identify, quantify, and characterize Revised: May 18, 2016 biomolecules like proteins from any number of biological conditions or sample types. As Accepted: July 8, 2016 instrumentation has advanced, and with the coupling of liquid chromatography (LC) for high- throughput LC-MS/MS, a proteomics experiment measuring hundreds to thousands of pro- teins/protein groups is now commonplace. While expert practitioners who best understand the operation of LC-MS systems tend to have strong backgrounds in physics and engineering, consumers of proteomics data and technology are not exposed to the physio-chemical principles underlying the information they seek. Since articles and reviews tend not to focus on bridging this divide, our goal here is to span this gap and translate MS ion physics into language intuitive to the general reader active in basic or applied biomedical research. Here, we visually describe what happens to ions as they enter and move around inside a mass spectrometer. We describe basic MS principles, including electric current, ion optics, ion traps, quadrupole mass filters, and Orbitrap FT-analyzers. -

Ion Trap Quantum Computing with Ca+Ions
Quantum Information Processing, Vol. 3, Nos. 1–5, October 2004 (© 2004) Ion Trap Quantum Computing with Ca+ Ions R. Blatt,1,2 H. Haffner,¨ 1 C. F. Roos,1 C. Becher,1,3 and F. Schmidt-Kaler1 Received April 6, 2004; accepted April 22, 2004 The scheme of an ion trap quantum computer is described and the implementation of quantum gate operations with trapped Ca+ ions is discussed. Quantum infor- mation processing with Ca+ ions is exemplified with several recent experiments investigating entanglement of ions. KEY WORDS: Quantum computation; trapped ions; ion trap quantum com- puter; Bell states; entanglement; controlled-NOT gate; state tomography; laser cooling. PACS: 03.67.Lx; 03.67.Mn; 32.80.Pj. 1. INTRODUCTION Quantum information processing was proposed and considered first by Feynman and Deutsch.(1,2) The requirements for a quantum processor are nowadays known as the DiVincenzo criteria.(3) Storing and processing quan- tum information requires: (i) scalable physical systems with well-defined qubits; which (ii) can be initialized; and have (iii) long lived quantum states in order to ensure long coherence times during the computational pro- cess. The necessity to coherently manipulate the stored quantum informa- tion requires: (iv) a set of universal gate operations between the qubits which must be implemented using controllable interactions of the quantum systems; and finally, to determine reliably the outcome of a quantum compu- tation (v) an efficient measurement procedure. In recent years, a large vari- ety of physical systems have been proposed and investigated for their use in 1Institut fur¨ Experimentalphysik, Universitat¨ Innsbruck, Technikerstrasse 25, A-6020 Innsbruck, Austria. -

Quadrupole and Ion Trap Mass Analysers and an Introduction to Resolution a Simple Definition of a Mass Spectrometer
Quadrupole and Ion Trap Mass Analysers and an introduction to Resolution A simple definition of a Mass Spectrometer ◗ A Mass Spectrometer is an analytical instrument that can separate charged molecules according to their mass–to–charge ratio. The mass spectrometer can answer the questions “what is in the sample” (qualitative structural information) and “how much is present” (quantitative determination) for a very wide range of samples at high sensitivity Components of a Mass Spectrometer Atmosphere Vacuum System Sample Ionisation Mass Data Detector Inlet Method Analyser System How are mass spectra produced ? ◗ Ions are produced in the source and are transferred into the mass analyser ◗ They are separated according to their mass/charge ratio in the mass analyser (e.g. Quadrupole, Ion Trap) ◗ Ions of the various m/z values exit the analyser and are ‘counted’ by the detector What is a Mass Spectrum ? ◗ A mass spectrum is the relative abundance of ions of different m/z produced in an ion source -a “chemical fingerprint” ◗ It contains – Molecular weight information (generally) – Structural Information (mostly) – Quantitative information What does a mass spectrum look like ? ◗ Electrospray mass spectrum of salbutamol + 100 MH 240 OH NH tBu HO Base peak HO % at m/z 240 241 0 60 80 100 120 140 160 180 200 220 240 260 280 300 What information do you need from the analysis ? ◗ Low or High Mass range ◗ Average or Monoisotopic mass (empirical) ◗ Accurate Mass ◗ Quantitation - precision, accuracy, selectivity ◗ Identification ◗ Structural Information -

Using Collision-Induced Dissociation Techniques to Constrain
Geophysical Research Abstracts Vol. 21, EGU2019-8406, 2019 EGU General Assembly 2019 © Author(s) 2019. CC Attribution 4.0 license. Using collision-induced dissociation techniques to constrain sensitivities of + ammonia chemical ionization mass spectrometry (NH4 − CIMS) to oxygenated organic compounds in the gas and particle phases Frank Keutsch (1,2,3), Alexander Zaytsev (1), Martin Breitenlechner (1), Abigail Koss (4), Christopher Lim (4), James Rowe (4), and Jesse Kroll (4) (1) School of Engineering and Applied Sciences, Harvard University, Cambridge, United States, (2) Department of Chemistry & Chemical Biology, Harvard University, Cambridge, United States, (3) Department of Earth and Planetary Sciences, Harvard University, Cambridge, United States, (4) Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, United States Chemical ionization mass spectrometry (CIMS) has become an important analytical tool for measurements of organic molecules in the atmosphere. A variety of reagent ions can be used to detect different classes of volatile or- ganic compounds (VOCs) and to analyze submicrometer particulate organic matter. However, detection efficiency and sensitivity of CIMS instruments depend critically on both the reagent ion and the measured sample molecule. We have developed a new CIMS instrument that is equipped with three similar corona discharge ion sources and currently can be operated in two different modes: (1) ligand switching reactions from adduct ions + + + NH4 •(H2O)n; (n = 0; 1; 2)(NH4 −CIMS) and (2) proton transfer reactions with H3O •(H2O)n; (n = 0; 1) ions (PTR − MS). We present a mass spectrometric voltage scanning procedure which is based on collision- induced dissociation that allows for the determination of the stability of detected ammonium-organic clusters.