An Introduction to Mass Spectrometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

About Magnetic Sector Mass Spec- Trometers
About Magnetic Sector Mass Spec- trometers Double-focusing magnetic sector mass spectrometers provide high sensitivity, high resolution, and a reproducibility that is unmatched in any other kind of mass analyzer. A double-focusing magnetic sector mass spectrometer has at least four components: . An ion source in which ions are formed and accelerated to energies of up to as high as 10 kilovolts. A magnetic sector with a magnetic field that exerts a force perpendicular to the ion mo- tion to deflect ions according to their momentum. An electric sector with an electric field that exerts a force perpendicular to the ion mo- tion to deflect ions according to their kinetic energy. A detector that produces a response that is proportional to the number of ions. Slits are placed in the ion path to define the positions and energies of the ions that strike the detector. In general, decreasing the slit widths increases the mass resolution but re- duces the number of ions that are detected. Additional electrostatic lenses are com- monly used to shape and deflect the ion beam to optimize peak shape and maximize ion beam transmission from the source to the detector. JEOL uses octapole and quadrupole focusing lenses to simplify the ion optical design. Collision chambers in the first field-free region (just after the ion source) and second field-free region (just after the magnet and before the electric sector) are used to induce ions to fragment in collision-induced-dissociation experiments (MS/MS). The term "double-focusing" refers to the fact that the combination of electric and mag- netic sectors focuses ions according to both direction and energy to provide higher reso- lution than can be obtained with a single magnetic sector. -

An Introduction to Isotopic Calculations John M
An Introduction to Isotopic Calculations John M. Hayes ([email protected]) Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA, 30 September 2004 Abstract. These notes provide an introduction to: termed isotope effects. As a result of such effects, the • Methods for the expression of isotopic abundances, natural abundances of the stable isotopes of practically • Isotopic mass balances, and all elements involved in low-temperature geochemical • Isotope effects and their consequences in open and (< 200°C) and biological processes are not precisely con- closed systems. stant. Taking carbon as an example, the range of interest is roughly 0.00998 ≤ 13F ≤ 0.01121. Within that range, Notation. Absolute abundances of isotopes are com- differences as small as 0.00001 can provide information monly reported in terms of atom percent. For example, about the source of the carbon and about processes in 13 13 12 13 atom percent C = [ C/( C + C)]100 (1) which the carbon has participated. A closely related term is the fractional abundance The delta notation. Because the interesting isotopic 13 13 fractional abundance of C ≡ F differences between natural samples usually occur at and 13F = 13C/(12C + 13C) (2) beyond the third significant figure of the isotope ratio, it has become conventional to express isotopic abundances These variables deserve attention because they provide using a differential notation. To provide a concrete the only basis for perfectly accurate mass balances. example, it is far easier to say – and to remember – that Isotope ratios are also measures of the absolute abun- the isotope ratios of samples A and B differ by one part dance of isotopes; they are usually arranged so that the per thousand than to say that sample A has 0.3663 %15N more abundant isotope appears in the denominator and sample B has 0.3659 %15N. -

Mass Spectrometry: Quadrupole Mass Filter
Advanced Lab, Jan. 2008 Mass Spectrometry: Quadrupole Mass Filter The mass spectrometer is essentially an instrument which can be used to measure the mass, or more correctly the mass/charge ratio, of ionized atoms or other electrically charged particles. Mass spectrometers are now used in physics, geology, chemistry, biology and medicine to determine compositions, to measure isotopic ratios, for detecting leaks in vacuum systems, and in homeland security. Mass Spectrometer Designs The first mass spectrographs were invented almost 100 years ago, by A.J. Dempster, F.W. Aston and others, and have therefore been in continuous development over a very long period. However the principle of using electric and magnetic fields to accelerate and establish the trajectories of ions inside the spectrometer according to their mass/charge ratio is common to all the different designs. The following description of Dempster’s original mass spectrograph is a simple illustration of these physical principles: The magnetic sector spectrograph PUMP F DD S S3 1 r S2 Fig. 1: Dempster’s Mass Spectrograph (1918). Atoms/molecules are first ionized by electrons emitted from the hot filament (F) and then accelerated towards the entrance slit (S1). The ions then follow a semicircular trajectory established by the Lorentz force in a uniform magnetic field. The radius of the trajectory, r, is defined by three slits (S1, S2, and S3). Ions with this selected trajectory are then detected by the detector D. How the magnetic sector mass spectrograph works: Equating the Lorentz force with the centripetal force gives: qvB = mv2/r (1) where q is the charge on the ion (usually +e), B the magnetic field, m is the mass of the ion and r the radius of the ion trajectory. -

Good Practice Guide for Isotope Ratio Mass Spectrometry, FIRMS (2011)
Good Practice Guide for Isotope Ratio Mass Spectrometry Good Practice Guide for Isotope Ratio Mass Spectrometry First Edition 2011 Editors Dr Jim Carter, UK Vicki Barwick, UK Contributors Dr Jim Carter, UK Dr Claire Lock, UK Acknowledgements Prof Wolfram Meier-Augenstein, UK This Guide has been produced by Dr Helen Kemp, UK members of the Steering Group of the Forensic Isotope Ratio Mass Dr Sabine Schneiders, Germany Spectrometry (FIRMS) Network. Dr Libby Stern, USA Acknowledgement of an individual does not indicate their agreement with Dr Gerard van der Peijl, Netherlands this Guide in its entirety. Production of this Guide was funded in part by the UK National Measurement System. This publication should be cited as: First edition 2011 J. F. Carter and V. J. Barwick (Eds), Good practice guide for isotope ratio mass spectrometry, FIRMS (2011). ISBN 978-0-948926-31-0 ISBN 978-0-948926-31-0 Copyright © 2011 Copyright of this document is vested in the members of the FIRMS Network. IRMS Guide 1st Ed. 2011 Preface A few decades ago, mass spectrometry (by which I mean organic MS) was considered a “black art”. Its complex and highly expensive instruments were maintained and operated by a few dedicated technicians and its output understood by only a few academics. Despite, or because, of this the data produced were amongst the “gold standard” of analytical science. In recent years a revolution occurred and MS became an affordable, easy to use and routine technique in many laboratories. Although many (rightly) applaud this popularisation, as a consequence the “black art” has been replaced by a “black box”: SAMPLES GO IN → → RESULTS COME OUT The user often has little comprehension of what goes on “under the hood” and, when “things go wrong”, the inexperienced operator can be unaware of why (or even that) the results that come out do not reflect the sample that goes in. -

Contents • Introduction to Proteomics and Mass Spectrometry
Contents • Introduction to Proteomics and Mass spectrometry - What we need to know in our quest to explain life - Fundamental things you need to know about mass spectrometry • Interfaces and ion sources - Electrospray ionization (ESI) - conventional and nanospray - Heated nebulizer atmospheric pressure chemical ionization - Matrix assisted laser desorption • Types of MS analyzers - Magnetic sector - Quadrupole - Time-of-flight - Hybrid - Ion trap In the next step in our quest to explain what is life The human genome project has largely been completed and many other genomes are surrendering to the gene sequencers. However, all this knowledge does not give us the information that is needed to explain how living cells work. To do that, we need to study proteins. In 2002, mass spectrometry has developed to the point where it has the capacity to obtain the "exact" molecular weight of many macromolecules. At the present time, this includes proteins up to 150,000 Da. Proteins of higher molecular weights (up to 500,000 Da) can also be studied by mass spectrometry, but with less accuracy. The paradigm for sequencing of peptides and identification of proteins has changed – because of the availability of the human genome database, peptides can be identified merely by their masses or by partial sequence information, often in minutes, not hours. This new capacity is shifting the emphasis of biomedical research back to the functional aspects of cell biochemistry, the expression of particular sets of genes and their gene products, the proteins of the cell. These are the new goals of the biological scientist: o to know which proteins are expressed in each cell, preferably one cell at a time o to know how these proteins are modified, information that cannot necessarily be deduced from the nucleotide sequence of individual genes. -

1.3.4 Atoms and Molecules Name Symbol Definition SI Unit
1.3.4 Atoms and molecules Name Symbol Definition SI unit Notes nucleon number, A 1 mass number proton number, Z 1 atomic number neutron number N N = A - Z 1 electron rest mass me kg (1) mass of atom, ma, m kg atomic mass 12 atomic mass constant mu mu = ma( C)/12 kg (1), (2) mass excess ∆ ∆ = ma - Amu kg elementary charge, e C proton charage Planck constant h J s Planck constant/2π h h = h/2π J s 2 2 Bohr radius a0 a0 = 4πε0 h /mee m -1 Rydberg constant R∞ R∞ = Eh/2hc m 2 fine structure constant α α = e /4πε0 h c 1 ionization energy Ei J electron affinity Eea J (1) Analogous symbols are used for other particles with subscripts: p for proton, n for neutron, a for atom, N for nucleus, etc. (2) mu is equal to the unified atomic mass unit, with symbol u, i.e. mu = 1 u. In biochemistry the name dalton, with symbol Da, is used for the unified atomic mass unit, although the name and symbols have not been accepted by CGPM. Chapter 1 - 1 Name Symbol Definition SI unit Notes electronegativity χ χ = ½(Ei +Eea) J (3) dissociation energy Ed, D J from the ground state D0 J (4) from the potential De J (4) minimum principal quantum n E = -hcR/n2 1 number (H atom) angular momentum see under Spectroscopy, section 3.5. quantum numbers -1 magnetic dipole m, µ Ep = -m⋅⋅⋅B J T (5) moment of a molecule magnetizability ξ m = ξB J T-2 of a molecule -1 Bohr magneton µB µB = eh/2me J T (3) The concept of electronegativity was intoduced by L. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -

Modern Mass Spectrometry
Modern Mass Spectrometry MacMillan Group Meeting 2005 Sandra Lee Key References: E. Uggerud, S. Petrie, D. K. Bohme, F. Turecek, D. Schröder, H. Schwarz, D. Plattner, T. Wyttenbach, M. T. Bowers, P. B. Armentrout, S. A. Truger, T. Junker, G. Suizdak, Mark Brönstrup. Topics in Current Chemistry: Modern Mass Spectroscopy, pp. 1-302, 225. Springer-Verlag, Berlin, 2003. Current Topics in Organic Chemistry 2003, 15, 1503-1624 1 The Basics of Mass Spectroscopy ! Purpose Mass spectrometers use the difference in mass-to-charge ratio (m/z) of ionized atoms or molecules to separate them. Therefore, mass spectroscopy allows quantitation of atoms or molecules and provides structural information by the identification of distinctive fragmentation patterns. The general operation of a mass spectrometer is: "1. " create gas-phase ions "2. " separate the ions in space or time based on their mass-to-charge ratio "3. " measure the quantity of ions of each mass-to-charge ratio Ionization sources ! Instrumentation Chemical Ionisation (CI) Atmospheric Pressure CI!(APCI) Electron Impact!(EI) Electrospray Ionization!(ESI) SORTING DETECTION IONIZATION OF IONS OF IONS Fast Atom Bombardment (FAB) Field Desorption/Field Ionisation (FD/FI) Matrix Assisted Laser Desorption gaseous mass ion Ionisation!(MALDI) ion source analyzer transducer Thermospray Ionisation (TI) Analyzers quadrupoles vacuum signal Time-of-Flight (TOF) pump processor magnetic sectors 10-5– 10-8 torr Fourier transform and quadrupole ion traps inlet Detectors mass electron multiplier spectrum Faraday cup Ionization Sources: Classical Methods ! Electron Impact Ionization A beam of electrons passes through a gas-phase sample and collides with neutral analyte molcules (M) to produce a positively charged ion or a fragment ion. -

Find the Molar Mass of Sodium Carbonate, Na 2CO3. Na 2 X
Moles and Molar Mass The mole is the "counting unit" used by chemists to indicate the number of atoms, ions, molecules, or formula units present in a particular chemical sample. The mole is similar to other counting units that you've used before....pair (2), dozen (12), and gross (144). One mole of a compound contains Avogadro's number (6.022 x 1023) of molecules (molecular compound) or formula units (ionic compound). The molar mass of a compound tells you the mass of 1 mole of that substance. In other words, it tells you the number of grams per mole of a compound. The units for molar mass are, therefore, grams/mole. To find the molar mass of a compound: 1. Use the chemical formula to determine the number of each type of atom present in the compound. 2. Multiply the atomic weight (from the periodic table) of each element by the number of atoms of that element present in the compound. 3. Add it all together and put units of grams/mole after the number. Example: Find the molar mass of sodium carbonate, Na2CO3. Na 2 x 23.0 = 46.0 C 1 x 12.0 = 12.0 O 3 x 16.0 = 48.0 molar = 106.0 g/mole mass For many (but not all) problems, you can simply round the atomic weights and the molar mass to the nearest 0.1 g/mole. HOWEVER, make sure that you use at least as many significant figures in your molar mass as the measurement with the fewest significant figures. -
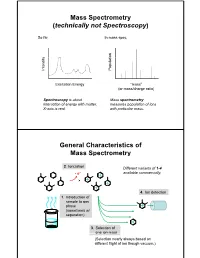
Mass Spectrometry (Technically Not Spectroscopy)
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

Glossary of Terms Used in Photochemistry, 3Rd Edition (IUPAC
Pure Appl. Chem., Vol. 79, No. 3, pp. 293–465, 2007. doi:10.1351/pac200779030293 © 2007 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY ORGANIC AND BIOMOLECULAR CHEMISTRY DIVISION* SUBCOMMITTEE ON PHOTOCHEMISTRY GLOSSARY OF TERMS USED IN PHOTOCHEMISTRY 3rd EDITION (IUPAC Recommendations 2006) Prepared for publication by S. E. BRASLAVSKY‡ Max-Planck-Institut für Bioanorganische Chemie, Postfach 10 13 65, 45413 Mülheim an der Ruhr, Germany *Membership of the Organic and Biomolecular Chemistry Division Committee during the preparation of this re- port (2003–2006) was as follows: President: T. T. Tidwell (1998–2003), M. Isobe (2002–2005); Vice President: D. StC. Black (1996–2003), V. T. Ivanov (1996–2005); Secretary: G. M. Blackburn (2002–2005); Past President: T. Norin (1996–2003), T. T. Tidwell (1998–2005) (initial date indicates first time elected as Division member). The list of the other Division members can be found in <http://www.iupac.org/divisions/III/members.html>. Membership of the Subcommittee on Photochemistry (2003–2005) was as follows: S. E. Braslavsky (Germany, Chairperson), A. U. Acuña (Spain), T. D. Z. Atvars (Brazil), C. Bohne (Canada), R. Bonneau (France), A. M. Braun (Germany), A. Chibisov (Russia), K. Ghiggino (Australia), A. Kutateladze (USA), H. Lemmetyinen (Finland), M. Litter (Argentina), H. Miyasaka (Japan), M. Olivucci (Italy), D. Phillips (UK), R. O. Rahn (USA), E. San Román (Argentina), N. Serpone (Canada), M. Terazima (Japan). Contributors to the 3rd edition were: A. U. Acuña, W. Adam, F. Amat, D. Armesto, T. D. Z. Atvars, A. Bard, E. Bill, L. O. Björn, C. Bohne, J. Bolton, R. Bonneau, H.