Us 9.211,237 B2 Cpc. A61k 8046
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

-

Penetrating Pharmaceutical Foam Eindringender Pharmazeutischer Schaum Mousse Pharmaceutique Pénétrante
(19) TZZ _T (11) EP 2 422 768 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/12 (2006.01) A61K 31/196 (2006.01) 15.04.2015 Bulletin 2015/16 A61K 31/167 (2006.01) A61Q 19/00 (2006.01) A61K 31/496 (2006.01) A61K 9/107 (2006.01) (2006.01) (2006.01) (21) Application number: 11190124.5 A61K 8/04 A61K 8/42 A61K 8/365 (2006.01) (22) Date of filing: 20.08.2004 (54) Penetrating pharmaceutical foam Eindringender pharmazeutischer Schaum Mousse pharmaceutique pénétrante (84) Designated Contracting States: • Raymond C Rowe, Paul J Sheskey and Marian E AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Quinn (Ed.): "Decyl oleate", Handbook of HU IE IT LI LU MC NL PL PT RO SE SI SK TR Pharmaceutical Excipients , 2011, pages 1-4, XP002666037, Retrieved from the Internet: URL: (30) Priority: 25.08.2003 US 497648 P http://www.medicinescomplete.com/mc/ex cipients/current/EXP-TD-c40-mn0001.htm?q=d (43) Date of publication of application: ecyloleate&t=search&ss=text&p=1#_hit 29.02.2012 Bulletin 2012/09 [retrieved on 2011-12-19] • Raymond C Rowe, Paul J Sheskey and Marian E (62) Document number(s) of the earlier application(s) in Quinn(Ed.): "Glyceryl Monooleate", Handbookof accordance with Art. 76 EPC: Pharmaceutical Excipients , 2011, pages 1-5, 04769356.9 / 1 663 148 XP002666038, Retrieved from the Internet: URL: http://www.medicinescomplete.com/mc/ex (73) Proprietor: Foamix Pharmaceuticals Ltd. -

Chemical UVR Absorbers
Chemical UVR Absorbers The names given in bold and used Diisopropyl methyl cinnamate Glyceryl ethyihexanoate dimethoxy- throughout this handbook are those of Empirical formula: cinnamate the International Nomenclature of C 6H22O2 Chemical names. Cosmetic Ingredients. Glyceryl octanoate dimethoxycinnamate; Chemical names: 2-propenoic acid, 3-(4-methoxyphenyl)-, 2-Propenoic acid, 3-12,4bis(1 diester with 1 ,3-dihydroxy-2-(2-ethyl-1 - methylethyphenyl-methyl ester; 2,5- oxohexyl)oxypropane diisopropyl methyl cinnamate _ lsoamyl-para-methoxycinnamate Ethyihexyl methoxycinnamate Empirical formula: Empirical formula: C151-12003 C 8H26O3 Chemical names: Cinnamates Chemical names: Amyl4-methoxycinnamate; isopentyl-4- 2-Ethylhexyl-4-methoxycin nam ate; methoxycinnamate; isopenlyl-para- Cinoxate 2-ethyl-hexyl-para-methoxycinnamate; methoxy-cinnamate; 3-(4-methoxyphenyl)- Empirical formula: para-methoxycinnamic acid, 2-ethylhexyl 2-propenoic acid, isopentyl ester Ci4HieO4 ester; 3-(4-methoxyphenyl)-2-propenoic acid, 2-ethylhexyl ester; octinoxate; octyl Trade names: Chemical names: methoxycinnamate; 2-propenoic acid, 3- Neo Heliopan type E 1000; Solarum AMC 2- Ethoxyothyl-para-methoxyci n nam ate; (4-methoxyphenyl)-2-ethylhexyl ester 2-propenoic acid, 3-(4-methoxyphery- para-A minobenzoic acids (PA BAs) 2-ethoxyethyl ester; 2-ethoxyethyl-4- Trade names: methoxycinnamate AEC Octyl Methoxycinnamate; Escalol Amyl dimethyl FABA 557; Eusolex 2292; Heliosol 3; Empirical formula: Trade names: Jeescreen OMC; Katoscreen OMC; Nec C14H21 NO2 Giv Tan F; Phiasol -

How to Use This Guide Dielectric Constant Table
Dielectric Constant Table.xls Dielectric Constant Table Dielectric Constant (k) is a number relating the ability of a material to carry alternating current to the ability of vacuum to carry alternating current. The capacitance created by the presence of the material is directly related to the Dielectric Constant of the material. Alphabetic Table A B C D Knowing the Dielectric Constant (k) of a material is needed to properly E F G H design and apply instruments such as level controls using radar, RF I J K L admittance, or capacitance technologies. There are also analytical M N O P reasons to know the (k) of a material. Q R S T U V W X How to use this guide Y Z # CLIPPER CONTROLS has compiled an extensive list of products with Dielectric Constants. Many of these Dielectric Constants are given at specific temperatures. If your product's temperature is significantly different from those listed there is a good chance that the Dielectric Constant may be different from the values listed. The products in this reference are listed in alphabetical order and are grouped in sections by the first letter of their name. Proper chemical names were used, and any trade names are the trademark of their respective owners. If you know the correct spelling of the name of the product you wish to review then use the "Search" feature on the web browser to locate the name in the list. You may also click on the letter from the alphabetical table to go directly to the beginning of that alphabetic section. -

ENGLISH POSITIVE LIST MATERIAL (ENG).Pdf
Attachment of Decree Number: SK15/Dir/LPPOM MUI/XI/19 HALAL POSITIVE LIST OF MATERIALS Halal Positive List of Materials consists of non critical materials, in terms of their halalness status, commonly used in prosessing industries. This material list was made based on the assessment of LPPOM MUI refer to the literature, abundance in nature, and consideration of commercially production scales. Companies that using materials listed in Halal Positive List of Materials will get advantages as follows: 1. In selection process of new materials, materials listed in Halal Positive List of Materials already get material approval from LPPOM MUI automatically prior to use. 2. In process of incoming material checking, materials listed in Halal Positive List of Materials does not require the suitability checking of the material name, manufacture's name and country of origin. 3. In product registration process, materials listed in Halal Positive List of Materials does not require supporting document. If the materials use trade name which is different with material name, the material specification document is still required. During the audit process, the auditor may check the supporting documents of material when necessary. Halal Positive List of Materials is given in the following table: Prohibited for Foods (BPOM CAS Chemical Name Regulation) Foods Cosmetics 673-84-7 2,6-Dimethyl-2,4,6-octatriene 91-57-6 2-Methylnaphthalene 1576-78-9 [(E)-hept-3-enyl] acetate 72214-23-4 7-acetoxy-3,7-dimethyl-octa-1,3- diene Page 3 of 192 2216-45-7 (4-methylphenyl)methyl -

Novel Salicylic Acid Analogs Induce a Potent Defense Response in Arabidopsis
University of South Carolina Scholar Commons Faculty Publications Biological Sciences, Department of 7-8-2019 Novel Salicylic Acid Analogs Induce a Potent Defense Response in Arabidopsis Ian Arthur Palmer University of South Carolina - Columbia Huan Chen University of South Carolina - Columbia, [email protected] Jian Chen University of South Carolina - Columbia Ming Chang University of South Carolina - Columbia, [email protected] Min Li University of South Carolina - Columbia, [email protected] See next page for additional authors Follow this and additional works at: https://scholarcommons.sc.edu/biol_facpub Part of the Biology Commons Publication Info Published in International Journal of Molecular Sciences, Volume 20, Issue 13, 2019, pages 1-15. © 2019, the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license . This Article is brought to you by the Biological Sciences, Department of at Scholar Commons. It has been accepted for inclusion in Faculty Publications by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Author(s) Ian Arthur Palmer, Huan Chen, Jian Chen, Ming Chang, Min Li, Fengquan Liu, and Zheng Qing Fu This article is available at Scholar Commons: https://scholarcommons.sc.edu/biol_facpub/253 International Journal of Molecular Sciences Article Novel Salicylic Acid Analogs Induce a Potent Defense Response in Arabidopsis Ian Arthur Palmer -

Dielectric Constant Chart
Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. F CONSTANT ABS RESIN, LUMP 2.4-4.1 ABS RESIN, PELLET 1.5-2.5 ACENAPHTHENE 70 3 ACETAL 70 3.6 ACETAL BROMIDE 16.5 ACETAL DOXIME 68 3.4 ACETALDEHYDE 41 21.8 ACETAMIDE 68 4 ACETAMIDE 180 59 ACETAMIDE 41 ACETANILIDE 71 2.9 ACETIC ACID 68 6.2 ACETIC ACID (36 DEGREES F) 36 4.1 ACETIC ANHYDRIDE 66 21 ACETONE 77 20.7 ACETONE 127 17.7 ACETONE 32 1.0159 ACETONITRILE 70 37.5 ACETOPHENONE 75 17.3 ACETOXIME 24 3 ACETYL ACETONE 68 23.1 ACETYL BROMIDE 68 16.5 ACETYL CHLORIDE 68 15.8 ACETYLE ACETONE 68 25 ACETYLENE 32 1.0217 ACETYLMETHYL HEXYL KETONE 66 27.9 ACRYLIC RESIN 2.7 - 4.5 ACTEAL 21 3.6 ACTETAMIDE 4 AIR 1 AIR (DRY) 68 1.000536 ALCOHOL, INDUSTRIAL 16-31 ALKYD RESIN 3.5-5 ALLYL ALCOHOL 58 22 ALLYL BROMIDE 66 7 ALLYL CHLORIDE 68 8.2 ALLYL IODIDE 66 6.1 ALLYL ISOTHIOCYANATE 64 17.2 ALLYL RESIN (CAST) 3.6 - 4.5 ALUMINA 9.3-11.5 ALUMINA 4.5 ALUMINA CHINA 3.1-3.9 ALUMINUM BROMIDE 212 3.4 ALUMINUM FLUORIDE 2.2 ALUMINUM HYDROXIDE 2.2 ALUMINUM OLEATE 68 2.4 1 Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. F CONSTANT ALUMINUM PHOSPHATE 6 ALUMINUM POWDER 1.6-1.8 AMBER 2.8-2.9 AMINOALKYD RESIN 3.9-4.2 AMMONIA -74 25 AMMONIA -30 22 AMMONIA 40 18.9 AMMONIA 69 16.5 AMMONIA (GAS?) 32 1.0072 AMMONIUM BROMIDE 7.2 AMMONIUM CHLORIDE 7 AMYL ACETATE 68 5 AMYL ALCOHOL -180 35.5 AMYL ALCOHOL 68 15.8 AMYL ALCOHOL 140 11.2 AMYL BENZOATE 68 5.1 AMYL BROMIDE 50 6.3 AMYL CHLORIDE 52 6.6 AMYL ETHER 60 3.1 AMYL FORMATE 66 5.7 AMYL IODIDE 62 6.9 AMYL NITRATE 62 9.1 AMYL THIOCYANATE 68 17.4 AMYLAMINE 72 4.6 AMYLENE 70 2 AMYLENE BROMIDE 58 5.6 AMYLENETETRARARBOXYLATE 66 4.4 AMYLMERCAPTAN 68 4.7 ANILINE 32 7.8 ANILINE 68 7.3 ANILINE 212 5.5 ANILINE FORMALDEHYDE RESIN 3.5 - 3.6 ANILINE RESIN 3.4-3.8 ANISALDEHYDE 68 15.8 ANISALDOXINE 145 9.2 ANISOLE 68 4.3 ANITMONY TRICHLORIDE 5.3 ANTIMONY PENTACHLORIDE 68 3.2 ANTIMONY TRIBROMIDE 212 20.9 ANTIMONY TRICHLORIDE 166 33 ANTIMONY TRICHLORIDE 5.3 ANTIMONY TRICODIDE 347 13.9 APATITE 7.4 2 Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. -

(12) Patent Application Publication (10) Pub. No.: US 2013/0156711 A1 Castro (43) Pub
US 2013 O156711A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0156711 A1 Castro (43) Pub. Date: Jun. 20, 2013 (54) TOPICAL SKIN CARE FORMULATION (52) U.S. Cl. CPC ................ A61K 8/375 (2013.01); A61O 19/08 (71) Applicant: Mary Kay Inc., Dallas, TX (US) (2013.01) USPC ............................................................ 424/59 (72) Inventor: Mauricio Castro, Carrolton, TX (US) (73) Assignee: Mary Kay Inc., Dallas, TX (US) (57) ABSTRACT (21) Appl. No.: 13/707, 176 Disclosed is a topical skin care composition, and method for its use, that includes 55 to 65% by weight of water, 15 to 20% (22) Filed: Dec. 6, 2012 by weight of a combination of 3,3,5-Trimethylcyclohexyl O O 2-hydroxybenzoate (homosalate), 1-(4-Methoxyphenyl)-3- Related U.S. Application Data (4-tert-butylphenyl)propane-1,3-dione (avobenzone), 2-eth (60) Provisional application No. 61/567,970, filed on Dec. ylhexyl 2-hydroxybenzoate (Octisalate), and 2-ethylhexyl 7, 2011. 2-cyano-3,3-diphenyl-2-propenoate (octocrylene), 3 to 4% by weight of distearlydimonium chloride, and 1 to 3% by Publication Classification weight of a combination of euterpe oleracea fruit extract, punica granatum sterols, caprooyltetrapeptide-3, tocopherol (51) Int. Cl. or tocopherol acetate, and niacinamide, wherein the compo A6 IK S/37 (2006.01) sition has a sun protection factor of at least 15 or about 15 to A61O 19/08 (2006.01) US 2013/0156711 A1 Jun. 20, 2013 TOPCAL SKN CARE FORMULATION Zoic acid (PABA), PABA esters (glyceryl PABA, amyldim ethyl PABA and octyldimethyl PABA), butyl PABA, ethyl CROSS REFERENCE TO RELATED PABA, ethyl dihydroxypropyl PABA, cinnamates (octyl APPLICATIONS methoxycinnamate, isoamyl p-methoxycinnamate, octyl 0001. -

(19) United States (12) Patent Application Publication (10) Pub
US 20070088508A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0088508 A1 Childs (43) Pub. Date: Apr. 19, 2007 (54) COCRYSTALLIZATION METHODS Related US. Application Data (60) Provisional application No. 60/721,115, ?led on Sep. (76) Inventor: Scott Childs, Atlanta, GA (US) 28, 2005. Publication Classi?cation Correspondence Address: (51) Int. Cl. FINNEGAN, HENDERSON, FARABOW, GARRETT & DUNNER G06F 19/00 (2006.01) (52) US. Cl. .............................................................. .. 702/19 LLP 901 NEW YORK AVENUE, NW (57) ABSTRACT WASHINGTON, DC 20001-4413 (US) Methods for preparing cocrystals Wherein solutions of active agents in suitable liquids are combined With solutions of (21) Appl. No.: 11/527,395 guests, both With and Without suitable liquids, in an interface region are described herein. Methods for analyzing the (22) Filed: Sep. 27, 2006 cocrystals are also described. Patent Application Publication Apr. 19, 2007 Sheet 1 0f 2 US 2007/0088508 A1 Patent Application Publication Apr. 19, 2007 Sheet 2 0f 2 US 2007/0088508 A1 US 2007/0088508 A1 Apr. 19, 2007 COCRYSTALLIZATION METHODS costs, and manufacturing method may be modi?ed by using [0001] This application claims the bene?t of priority to a cocrystal rather than the active agent alone, or as a salt. provisional application No. 60/721,115, ?led on Sep. 28, [0006] An active agent can be screened for possible coc 2005, the contents of Which are incorporated by reference rystals Where polymorphic forms, hydrates, or solvates are herein. especially problematic. For example, a neutral compound that can only be isolated as amorphous material could be [0002] Cocrystals are crystals that contain tWo or more cocrystalliZed. -

WO 2017/058594 Al 6 April 2017 (06.04.2017) W P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/058594 Al 6 April 2017 (06.04.2017) W P O PCT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 409/12 (2006.01) A23L 33/10 (201 6.01) kind of national protection available): AE, AG, AL, AM, A61K 31/4155 (2006.01) A61Q 19/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 8/49 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/US20 16/052777 KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (22) International Filing Date: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 2 1 September 2016 (21 .09.201 6) OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (26) Publication Language: English ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 62/236,080 1 October 201 5 (01. 10.2015) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: SENOMYX, INC. -

(12) United States Patent (10) Patent No.: US 7,700,076 B2 Tamarkin Et Al
US007700076B2 (12) United States Patent (10) Patent No.: US 7,700,076 B2 Tamarkin et al. (45) Date of Patent: *Apr. 20, 2010 (54) PENETRATING PHARMACEUTICAL FOAM 3,923,970 A 12/1975 Breuer 3,929,985 A 12/1975 Webb, Jr. (75) Inventors: Dov Tamarkin, Maccabim (IL); Doron 3,959,160 A 5, 1976 Horsler et al. Friedman, Karmei Yosef (IL); Meir 3,962,150 A 6, 1976 Viola Eini, Ness Ziona (IL) 3,997.467 A 12/1976 Jederstrom et al. 4,001,442 A 1/1977 Stahlberger et al. (73) Assignee: Foamix, Ltd., Ness Ziona (IT) 4,019,657 A 4/1977 Spitzer et al. 4,145,411 A 3, 1979 Mende (*) Notice: Subject to any disclaimer, the term of this 4,241,149 A 12/1980 Labes et al. patent is extended or adjusted under 35 4,268,499 A 5/1981 Keil ............................ 424,68 U.S.C. 154(b) by 1425 days. 4,271,149 A 6, 1981 Winicov et al. 4,292,326 A 9, 1981 Nazzaro-Porro et al. This patent is Subject to a terminal dis 4,299,826 A 11/1981 Luedders claimer. 4.323,694. A 4, 1982 Scala, Jr. 4,386,104 A 5/1983 Nazzaro-Porro (21) Appl. No.: 10/922,358 4,447.486 A 5/1984 Hoppe et al. 4,508,705 A 4, 1985 Chaudhuri et al. (22) Filed: Aug. 20, 2004 4,529,601 A 7/1985 Broberg et al. 4,627,973 A 12/1986 Moran et al. (65) Prior Publication Data 4,628,063 A 12/1986 Haines et al. -
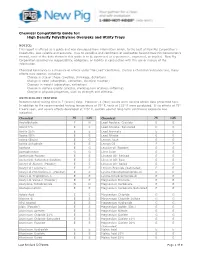
Chemical Compatibility Guide For: High Density Polyethylene Overpaks and Utility Trays
Chemical Compatibility Guide for: High Density Polyethylene Overpaks and Utility Trays NOTICE: This report is offered as a guide and was developed from information which, to the best of New Pig Corporation’s knowledge, was reliable and accurate. Due to variables and conditions of application beyond New Pig Corporation’s control, none of the data shown in this guide is to be construed as a guarantee, expressed, or implied. New Pig Corporation assumes no responsibility, obligation, or liability in conjunction with the use or misuse of the information. Chemical Resistance is a measure of effects under “No Load” conditions. During a chemical resistance test, many effects may appear, including: Change in size or shape (swelling, shrinkage, distortion) Change in color (absorption, extraction, chemical reaction) Changes in weight (absorption, extraction) Change in surface quality (crazing, cracking, loss of gloss, softening) Change in physical properties, such as strength and stiffness ASTM D43-60T TESTING Recommended testing time is 7 (seven) days. However, 4 (four) weeks were used to obtain data presented here. In addition to the recommended testing temperature of 75° F, tests at 125° F were conducted. If no effects at 75° F were seen, and severe effects developed at 125° F, caution against long-term continuous exposure was indicated. Chemical 75˚ 125˚ Chemical 75˚ 125˚ Acetaldehyde F N Lead Acetate: Crystals E E Acetic 5% E E Lead Acetate: Saturated E E Acetic 25% E E Lead Arsenate E E Acetic 50% E E Lead Nitrate E E Acetic Glacial E G Lemon