And Other Submersed Aquatic Macrophytes in Lake Bisina and Other Ugandan Lakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"A Revision of the Freshwater Crabs of Lake Kivu, East Africa."
Northern Michigan University NMU Commons Journal Articles FacWorks 2011 "A revision of the freshwater crabs of Lake Kivu, East Africa." Neil Cumberlidge Northern Michigan University Kirstin S. Meyer Follow this and additional works at: https://commons.nmu.edu/facwork_journalarticles Part of the Biology Commons Recommended Citation Cumberlidge, Neil and Meyer, Kirstin S., " "A revision of the freshwater crabs of Lake Kivu, East Africa." " (2011). Journal Articles. 30. https://commons.nmu.edu/facwork_journalarticles/30 This Journal Article is brought to you for free and open access by the FacWorks at NMU Commons. It has been accepted for inclusion in Journal Articles by an authorized administrator of NMU Commons. For more information, please contact [email protected],[email protected]. This article was downloaded by: [Cumberlidge, Neil] On: 16 June 2011 Access details: Access Details: [subscription number 938476138] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37- 41 Mortimer Street, London W1T 3JH, UK Journal of Natural History Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713192031 The freshwater crabs of Lake Kivu (Crustacea: Decapoda: Brachyura: Potamonautidae) Neil Cumberlidgea; Kirstin S. Meyera a Department of Biology, Northern Michigan University, Marquette, Michigan, USA Online publication date: 08 June 2011 To cite this Article Cumberlidge, Neil and Meyer, Kirstin S.(2011) 'The freshwater crabs of Lake Kivu (Crustacea: Decapoda: Brachyura: Potamonautidae)', Journal of Natural History, 45: 29, 1835 — 1857 To link to this Article: DOI: 10.1080/00222933.2011.562618 URL: http://dx.doi.org/10.1080/00222933.2011.562618 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. -

CHIRONOMUS Newsletter on Chironomidae Research
CHIRONOMUS Newsletter on Chironomidae Research No. 25 ISSN 0172-1941 (printed) 1891-5426 (online) November 2012 CONTENTS Editorial: Inventories - What are they good for? 3 Dr. William P. Coffman: Celebrating 50 years of research on Chironomidae 4 Dear Sepp! 9 Dr. Marta Margreiter-Kownacka 14 Current Research Sharma, S. et al. Chironomidae (Diptera) in the Himalayan Lakes - A study of sub- fossil assemblages in the sediments of two high altitude lakes from Nepal 15 Krosch, M. et al. Non-destructive DNA extraction from Chironomidae, including fragile pupal exuviae, extends analysable collections and enhances vouchering 22 Martin, J. Kiefferulus barbitarsis (Kieffer, 1911) and Kiefferulus tainanus (Kieffer, 1912) are distinct species 28 Short Communications An easy to make and simple designed rearing apparatus for Chironomidae 33 Some proposed emendations to larval morphology terminology 35 Chironomids in Quaternary permafrost deposits in the Siberian Arctic 39 New books, resources and announcements 43 Finnish Chironomidae 47 Chironomini indet. (Paratendipes?) from La Selva Biological Station, Costa Rica. Photo by Carlos de la Rosa. CHIRONOMUS Newsletter on Chironomidae Research Editors Torbjørn EKREM, Museum of Natural History and Archaeology, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway Peter H. LANGTON, 16, Irish Society Court, Coleraine, Co. Londonderry, Northern Ireland BT52 1GX The CHIRONOMUS Newsletter on Chironomidae Research is devoted to all aspects of chironomid research and aims to be an updated news bulletin for the Chironomidae research community. The newsletter is published yearly in October/November, is open access, and can be downloaded free from this website: http:// www.ntnu.no/ojs/index.php/chironomus. Publisher is the Museum of Natural History and Archaeology at the Norwegian University of Science and Technology in Trondheim, Norway. -

Dear Colleagues
NEW RECORDS OF CHIRONOMIDAE (DIPTERA) FROM CONTINENTAL FRANCE Joel Moubayed-Breil Applied ecology, 10 rue des Fenouils, 34070-Montpellier, France, Email: [email protected] Abstract Material recently collected in Continental France has allowed me to generate a list of 83 taxa of chironomids, including 37 new records to the fauna of France. According to published data on the chironomid fauna of France 718 chironomid species are hitherto known from the French territories. The nomenclature and taxonomy of the species listed are based on the last version of the Chironomidae data in Fauna Europaea, on recent revisions of genera and other recent publications relevant to taxonomy and nomenclature. Introduction French territories represent almost the largest Figure 1. Major biogeographic regions and subregions variety of aquatic ecosystems in Europe with of France respect to both physiographic and hydrographic aspects. According to literature on the chironomid fauna of France, some regions still are better Sites and methodology sampled then others, and the best sampled areas The identification of slide mounted specimens are: The northern and southern parts of the Alps was aided by recent taxonomic revisions and keys (regions 5a and 5b in figure 1); western, central to adults or pupal exuviae (Reiss and Säwedal and eastern parts of the Pyrenees (regions 6, 7, 8), 1981; Tuiskunen 1986; Serra-Tosio 1989; Sæther and South-Central France, including inland and 1990; Soponis 1990; Langton 1991; Sæther and coastal rivers (regions 9a and 9b). The remaining Wang 1995; Kyerematen and Sæther 2000; regions located in the North, the Middle and the Michiels and Spies 2002; Vårdal et al. -

Chironominae 8.1
CHIRONOMINAE 8.1 SUBFAMILY CHIRONOMINAE 8 DIAGNOSIS: Antennae 4-8 segmented, rarely reduced. Labrum with S I simple, palmate or plumose; S II simple, apically fringed or plumose; S III simple; S IV normal or sometimes on pedicel. Labral lamellae usually well developed, but reduced or absent in some taxa. Mentum usually with 8-16 well sclerotized teeth; sometimes central teeth or entire mentum pale or poorly sclerotized; rarely teeth fewer than 8 or modified as seta-like projections. Ventromental plates well developed and usually striate, but striae reduced or vestigial in some taxa; beard absent. Prementum without dense brushes of setae. Body usually with anterior and posterior parapods and procerci well developed; setal fringe not present, but sometimes with bifurcate pectinate setae. Penultimate segment sometimes with 1-2 pairs of ventral tubules; antepenultimate segment sometimes with lateral tubules. Anal tubules usually present, reduced in brackish water and marine taxa. NOTESTES: Usually the most abundant subfamily (in terms of individuals and taxa) found on the Coastal Plain of the Southeast. Found in fresh, brackish and salt water (at least one truly marine genus). Most larvae build silken tubes in or on substrate; some mine in plants, dead wood or sediments; some are free- living; some build transportable cases. Many larvae feed by spinning silk catch-nets, allowing them to fill with detritus, etc., and then ingesting the net; some taxa are grazers; some are predacious. Larvae of several taxa (especially Chironomus) have haemoglobin that gives them a red color and the ability to live in low oxygen conditions. With only one exception (Skutzia), at the generic level the larvae of all described (as adults) southeastern Chironominae are known. -

Hunters Or Farmers? Microbiome Characteristics Help Elucidate the Diet Composition in an Aquatic Carnivorous Plant
Sirová et al. Microbiome (2018) 6:225 https://doi.org/10.1186/s40168-018-0600-7 RESEARCH Open Access Hunters or farmers? Microbiome characteristics help elucidate the diet composition in an aquatic carnivorous plant Dagmara Sirová1,2*† ,Jiří Bárta2†, Karel Šimek1,2, Thomas Posch3,Jiří Pech2, James Stone4,5, Jakub Borovec1, Lubomír Adamec6 and Jaroslav Vrba1,2 Abstract Background: Utricularia are rootless aquatic carnivorous plants which have recently attracted the attention of researchers due to the peculiarities of their miniaturized genomes. Here, we focus on a novel aspect of Utricularia ecophysiology—the interactions with and within the complex communities of microorganisms colonizing their traps and external surfaces. Results: Bacteria, fungi, algae, and protozoa inhabit the miniature ecosystem of the Utricularia trap lumen and are involved in the regeneration of nutrients from complex organic matter. By combining molecular methods, microscopy, and other approaches to assess the trap-associated microbial community structure, diversity, function, as well as the nutrient turn-over potential of bacterivory, we gained insight into the nutrient acquisition strategies of the Utricularia hosts. Conclusions: We conclude that Utricularia traps can, in terms of their ecophysiological function, be compared to microbial cultivators or farms, which center around complex microbial consortia acting synergistically to convert complex organic matter, often of algal origin, into a source of utilizable nutrients for the plants. Keywords: Algae, Bacteria, Ciliate bacterivory, Digestive mutualism, Fungi, Herbivory, Nutrient turnover, Plant– microbe interactions, Protists, Utricularia traps Background microbial communities clearly play a significant role in Plant-associated microorganisms have long been plant ecophysiology, but many of the underlying mech- recognized as key partners in enhancing plant nutrient anisms governing these looser associations still remain acquisition, mitigating plant stress, promoting growth, unexplored [2]. -

Biological Monitoring of Surface Waters in New York State, 2019
NYSDEC SOP #208-19 Title: Stream Biomonitoring Rev: 1.2 Date: 03/29/19 Page 1 of 188 New York State Department of Environmental Conservation Division of Water Standard Operating Procedure: Biological Monitoring of Surface Waters in New York State March 2019 Note: Division of Water (DOW) SOP revisions from year 2016 forward will only capture the current year parties involved with drafting/revising/approving the SOP on the cover page. The dated signatures of those parties will be captured here as well. The historical log of all SOP updates and revisions (past & present) will immediately follow the cover page. NYSDEC SOP 208-19 Stream Biomonitoring Rev. 1.2 Date: 03/29/2019 Page 3 of 188 SOP #208 Update Log 1 Prepared/ Revision Revised by Approved by Number Date Summary of Changes DOW Staff Rose Ann Garry 7/25/2007 Alexander J. Smith Rose Ann Garry 11/25/2009 Alexander J. Smith Jason Fagel 1.0 3/29/2012 Alexander J. Smith Jason Fagel 2.0 4/18/2014 • Definition of a reference site clarified (Sect. 8.2.3) • WAVE results added as a factor Alexander J. Smith Jason Fagel 3.0 4/1/2016 in site selection (Sect. 8.2.2 & 8.2.6) • HMA details added (Sect. 8.10) • Nonsubstantive changes 2 • Disinfection procedures (Sect. 8) • Headwater (Sect. 9.4.1 & 10.2.7) assessment methods added • Benthic multiplate method added (Sect, 9.4.3) Brian Duffy Rose Ann Garry 1.0 5/01/2018 • Lake (Sect. 9.4.5 & Sect. 10.) assessment methods added • Detail on biological impairment sampling (Sect. -
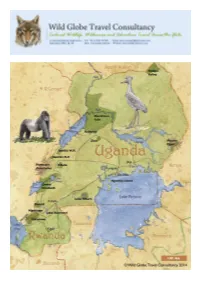
Uganda and Rwanda
Uganda: The Long Way Round - 50 Days Major Destinations Entebbe - Lake Victoria - Ngamba Island - Jinja - Mabira Forest Reserve - Sipi Falls - Mount Elgon National Park - Kidepo Valley National Park - Murchison Falls National Park - Budongo Forest Reserve - Ziwa Rhino Sanctuary - Semliki Wildlife Reserve - Semliki National Park - Kibale National Park - Bigodi Wetlands Sanctuary - Rwenzori Mountains National Park - Queen Elizabeth National Park - Bwindi Impenetrable National Park - Mgahinga Gorilla National Park - Volcanoes National Park - Kigali - Lake Bunyonyi - Lake Mburo National Park - Entebbe Tour Highlights and Activities Uganda’s geography is very different than its East Africa neighbours Kenya and Tanzania, as it has far more of the lush forested areas that flourish across the ‘equatorial forest belt’ of central and western Africa. Consequently it does not have the vast rolling savannahs of Kenya and particularly Tanzania, or the huge proliferation of plains animals that these countries are famous for. It is also only now recovering from the widespread poaching that went unchecked during years of violent conflict and political turmoil, which resulted in the destruction of massive animal populations and the local extinction of the rhino and wild dog. Although poaching does still occur in Uganda, as it sadly does all over Africa, the wildlife is now receiving a serious level of protection and is recovering remarkably well in most areas. The 2012 Uganda Wildlife Authority figures fully support this recovery, as the populations of many large species have more than doubled since the previous census in 1999, with the number of impala rising from around 1,600 to over 35,000. Elephant, buffalo, giraffe, zebra, hippo and waterbuck populations have all increased significantly, confirming what those of us visiting regularly already knew, the animals are returning and Uganda is once again featuring as one of the top wildlife destinations on this or any other continent. -

Ohio EPA Macroinvertebrate Taxonomic Level December 2019 1 Table 1. Current Taxonomic Keys and the Level of Taxonomy Routinely U
Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Table 1. Current taxonomic keys and the level of taxonomy routinely used by the Ohio EPA in streams and rivers for various macroinvertebrate taxonomic classifications. Genera that are reasonably considered to be monotypic in Ohio are also listed. Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Species Pennak 1989, Thorp & Rogers 2016 Porifera If no gemmules are present identify to family (Spongillidae). Genus Thorp & Rogers 2016 Cnidaria monotypic genera: Cordylophora caspia and Craspedacusta sowerbii Platyhelminthes Class (Turbellaria) Thorp & Rogers 2016 Nemertea Phylum (Nemertea) Thorp & Rogers 2016 Phylum (Nematomorpha) Thorp & Rogers 2016 Nematomorpha Paragordius varius monotypic genus Thorp & Rogers 2016 Genus Thorp & Rogers 2016 Ectoprocta monotypic genera: Cristatella mucedo, Hyalinella punctata, Lophopodella carteri, Paludicella articulata, Pectinatella magnifica, Pottsiella erecta Entoprocta Urnatella gracilis monotypic genus Thorp & Rogers 2016 Polychaeta Class (Polychaeta) Thorp & Rogers 2016 Annelida Oligochaeta Subclass (Oligochaeta) Thorp & Rogers 2016 Hirudinida Species Klemm 1982, Klemm et al. 2015 Anostraca Species Thorp & Rogers 2016 Species (Lynceus Laevicaudata Thorp & Rogers 2016 brachyurus) Spinicaudata Genus Thorp & Rogers 2016 Williams 1972, Thorp & Rogers Isopoda Genus 2016 Holsinger 1972, Thorp & Rogers Amphipoda Genus 2016 Gammaridae: Gammarus Species Holsinger 1972 Crustacea monotypic genera: Apocorophium lacustre, Echinogammarus ischnus, Synurella dentata Species (Taphromysis Mysida Thorp & Rogers 2016 louisianae) Crocker & Barr 1968; Jezerinac 1993, 1995; Jezerinac & Thoma 1984; Taylor 2000; Thoma et al. Cambaridae Species 2005; Thoma & Stocker 2009; Crandall & De Grave 2017; Glon et al. 2018 Species (Palaemon Pennak 1989, Palaemonidae kadiakensis) Thorp & Rogers 2016 1 Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Informal grouping of the Arachnida Hydrachnidia Smith 2001 water mites Genus Morse et al. -

SET III Living Together in East Africa
SET III Living Together in East Africa. Major Resources of East Africa. Meaning of resources/Examples. A resource is a feature in the environment that man uses to satisfy their /his needs. Types of natural resources. Renewable resources. Renewable resources are resources that can be replaced naturally once they are over- exploited. Non-renewable resources are resources that cannot be replace naturally once they are over-used or exhausted. Examples of renewable resources. • Plants • Animals • Water bodies • Land • Climate /rainfall/sunshine Examples of non-renewable resources • Minerals • Fossils fuel i.e. coal, oil, natural, gas Land • Land is the part of the earth that is not covered by water • Land supports most resources in the environment. 1 Importance of land • Land provides space for building houses / settlement. • Land is where crops are grown. • Land provides space for burying the dead. • Land provides space for grazing animals. • Minerals are mined from land. Problems facing land. • Dumping of garbage and toxic materials on land. • Over-cultivation • Deforestation • Land fragmentation • Soil erosion Possible solutions to some of the above problems. • Garbage should be used for other purposes like generation of biogas. • People should be encouraged to grow fodder crops for animals. • People should be encouraged to use manure and fertilizer. • Farmers should terrace their land to control soil erosion. • Educate the people about the benefits of re-afforestation. Note: There are things that people make to meet their needs and they are called human made resources. Examples include; - Electricity - Clothes - Shoes - Mobile phones - Books - Buildings - Vehicles - Drugs - Roads 2 Activity 1. What are natural resources? ………………………………………………………………………………………………………………………………… 2. -

Discriminating Permanent from Temporary Rivers with Traits of Chironomid Genera
Ann. Limnol. - Int. J. Lim. 53 (2017) 161–174 Available online at: Ó EDP Sciences, 2017 www.limnology-journal.org DOI: 10.1051/limn/2017004 Discriminating permanent from temporary rivers with traits of chironomid genera So´nia R. Q. Serra1*, Manuel A. S. Grac¸a1, Sylvain Dole´dec2 and Maria Joa˜ o Feio1 1 Department of Life Sciences, MARE – Marine and Environmental Sciences Centre, University of Coimbra, Largo Marqueˆ sde Pombal, 3004-517 Coimbra, Portugal 2 University Lyon 1, UMR 5023 LEHNA, Biodiversite´des Ecosyste` mes Lotiques, Baˆ t Forel, 69622 Villeurbanne Cedex, France Received 18 July 2016; Accepted 06 February 2017 Abstract – Chironomidae are diverse and present a wide variety of ecological preferences. Thus, they have high potential in establishing reference conditions for river bioassessment and in providing functional information, especially when other macroinvertebrates are poorly represented. However, because of taxo- nomic difficulties and poor knowledge of traits, they are neglected in bioassessment programmes and kept at coarser taxonomic levels, reducing the discrimination power of invertebrate-based diagnostic tools. Here, we compared the efficiency of Chironomidae at the subfamily and genus levels and their biological traits (Eltonian and morphological) in the distinction between permanent (medium altitude and lowland) and temporary Mediterranean streams. We established a priori predictions on the expected Chironomidae trait categories in each stream type, conferring the best adaptations to particular environmental constraints. Genus composition (not subfamily) and respective trait categories differed among the three stream types. Both biological traits identified differences between stream types. Among Eltonian traits, diapause stages seg- regated permanent medium altitude from lowland and temporary stream assemblages, reflecting adaptations to temperature and flow regime variations. -

DNA Barcoding
Full-time PhD studies of Ecology and Environmental Protection Piotr Gadawski Species diversity and origin of non-biting midges (Chironomidae) from a geologically young lake PhD Thesis and its old spring system Performed in Department of Invertebrate Zoology and Hydrobiology in Institute of Ecology and Environmental Protection Różnorodność gatunkowa i pochodzenie fauny Supervisor: ochotkowatych (Chironomidae) z geologicznie Prof. dr hab. Michał Grabowski młodego jeziora i starego systemu źródlisk Auxiliary supervisor: Dr. Matteo Montagna, Assoc. Prof. Łódź, 2020 Łódź, 2020 Table of contents Acknowledgements ..........................................................................................................3 Summary ...........................................................................................................................4 General introduction .........................................................................................................6 Skadar Lake ...................................................................................................................7 Chironomidae ..............................................................................................................10 Species concept and integrative taxonomy .................................................................12 DNA barcoding ...........................................................................................................14 Chapter I. First insight into the diversity and ecology of non-biting midges (Diptera: Chironomidae) -

Backparkers-Brochure.Pdf
Accommodation Prices 2018. Accomodation rates in $(USD) Per person per night I N D I Accomodation type Bed only Bed & Breakfast Half Board Full Board B W Self Camping $10PP $15PP $25PP $35PP Min Dormitory (4 Beds) $20PP $25PP $35PP $45PP Big Dormitory (6 Beds and above) $15PP $20PP $30PP $40PP Rent a mobile tent with beddings $15PP $20PP $30PP $40PP B Rent a mobile tent without beddings (pp) $12PP $17PP $27PP $35PP a e ck dg Twin Bed/Double Rooms with shared Bathroom $25PP $30PP $40PP $50PP packers Lo Single Bed room with shared Bathroom $30PP $35PP $45PP $55PP Twin Bed/Double Rooms self contained $40PP $45PP $55PP $65PP Single Bed Room self contained $45PP $50PP $60PP $70PP BWINDI BACKPACKERS LODGE Twin/Double Bedroom cottage $50PP $55PP $65PP $75PP Single Bedroom cottage $60PP $65PP $75PP $85PP LAST MINUTE GORILLA TRACKING PERMITS AND 3 Bedroom cottage $45PP $50PP $60PP $70PP ACTIVE NYIRAGONGO VOLCANO TREKKING/HIKING PERMITS AVAILABLE We accept payment by the following cards at no extra cost or by Mobile money on any of the following telephone numbers; To all clients paying for bed +256772661854, +256752661854, +256774883710 and breakfast, half board and full board, we serve full continental breakfast & for meals they are 3 course meals. Alcarte menu available for single course meals. Note:Prices can change without prio notice Note: half board includes Bed, You can pay by card on our website at no extra fees or Breakfast and Lunch or Dinner request for a card payment link by e-mail.. while full board includes Breakfast, Lunch & Dinner, It Half the price double the Fun!! excludes drinks Lodge shuttle: Departs from Kabale at 10:30am & 4:00pm.