Permethrin Resistance in Aedes Aegypti Affects Aspects of Vectorial Capacity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TITLE: Lindane and Other Treatments for Lice and Scabies: a Review of Clinical Effectiveness and Safety
TITLE: Lindane and Other Treatments for Lice and Scabies: A Review of Clinical Effectiveness and Safety DATE: 11 June 2010 CONTEXT AND POLICY ISSUES: Head lice infestation (Pediculosis capitis) affects millions of children and adults worldwide each year.1 Direct head-to-head contact is the most common mode of transmission.2 The highest prevalence of infestation occurs in school aged children aged three to eleven years, with girls being more commonly affected than boys.1,2 Although head lice are not generally associated with serious morbidity, they are responsible for significant social embarrassment and lost productivity in schools or offices.1 Scabies, an infestation of the skin by the mite Sarcoptes scabiei, represents a common public health concern particularly in overcrowded communities with a high prevalence of poverty.3 Scabies is transmitted by close-person contact and occasionally by clothing or linens.3 Complications include secondary bacterial infections and post-streptococcal glomerulonephritis.3 Topical products available in Canada for the treatment of head lice and scabies are presented in Appendix 1 and Appendix 2. Insecticidal agents such as permethrin and lindane have historically been considered the standard treatments for head lice and scabies.2,3 Toxicity is low following topical administration of permethrin due to minimal percutaneous absorption.4 However, several jurisdictions have banned lindane due to concerns of neurotoxicity and bone marrow suppression, as well as potential negative effects on the environment (contamination of waste water).5 Furthermore, widespread use of permethrin, pyrethrins/piperonyl butoxide, and lindane has led to resistance and higher rates of treatment failure.6 Resistance patterns and rates to these agents in Canada have not yet been studied.6 Due to concerns surrounding resistance and neurotoxicity, patients and caregivers have searched for alternative treatments. -
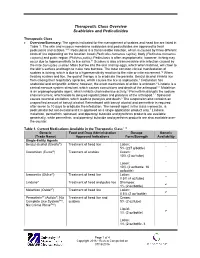
Therapeutic Class Overview Scabicides and Pediculicides
Therapeutic Class Overview Scabicides and Pediculicides Therapeutic Class Overview/Summary: The agents indicated for the management of scabies and head lice are listed in Table 1. The skin and mucous membrane scabicides and pediculicides are approved to treat pediculosis and scabies.1-10 Pediculosis is a transmissible infection, which is caused by three different kinds of lice depending on the location: head (Pediculus humanus capitis), body (Pediculus humanus corporis) and pubic region (Phthirus pubis). Pediculosis is often asymptomatic; however, itching may occur due to hypersensitivity to lice saliva.11 Scabies is also a transmissible skin infection caused by the mite Sarcoptes scabiei. Mites burrow into the skin and lay eggs, which when hatched, will crawl to the skin’s surface and begin to make new burrows. The most common clinical manifestation of scabies is itching, which is due to a hypersensitivity reaction to the mite or mite excrement.12 When treating scabies and lice, the goal of therapy is to eradicate the parasite. Benzyl alcohol inhibits lice from closing their respiratory spiracles, which causes the lice to asphyxiate.3 Crotamiton has scabicidal and antipruritic actions; however, the exact mechanism of action is unknown.4 Lindane is a central nervous system stimulant, which causes convulsions and death of the arthropod.1,2 Malathion is an organophosphate agent, which inhibits cholinesterase activity.5 Permethrin disrupts the sodium channel current, which leads to delayed repolarization and paralysis of the arthropod.1,2 Spinosad causes neuronal excitation, which leads to paralysis and death.6 The suspension also contains an unspecified amount of benzyl alcohol. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Household Insects – Homeowners ` CAUTION: All Insecticides Are Toxic to Some Degree; Therefore, Care Should Be Exercised in Their Use
Household Insects – Homeowners ` CAUTION: All insecticides are toxic to some degree; therefore, care should be exercised in their use. The manufacturer’s directions on the label in the use of the material must be followed explicitly. Insect Threats Insecticides and Treatment* Remarks Ants Feed on foods and Baits (active ingredient and Remove food and clean up the area. Place (several may damage product): bait where ants occur or congregate. May species) clothing; may also sodium tetraborate decahydrate use several different baits at the same time sting, causing severe (Amdro Kills Ants Liquid Bait, Terro to discover one that ants will consume. reaction to some Liquid Ant Baits); Care should be taken not to contaminate people. hydramethylnon (Amdro Kills Ants foodstuffs. Also treat nests in yard. Follow Bait Stations and Stakes); label. orthoboric acid (Terro Perimeter Ant Bait); fipronil (Combat Max Ant Killing Bait Stations and Gel); abamectin (Raid Max Double Control Ant Baits, Raid Ant Baits III); dinotefuran (Hot Shot Ultra Clear Roach & Ant Gel Bait, Hot Shot Ultra Liquid Ant Bait); spinosad (Ortho Home Defense Liquid Ant Bait); thiamethoxam (Raid Precision Placement Ant Bait Gel) Crack and crevices: Follow label. prallethrin, esfenvalerate, pyrethrins, pyrethrum, permethrin, tetra- methrin, phenothrin, beta-cyfluthrin, cyfluthrin Indoor space: prallethrin, esfenvalerate, pyrethrins, pyrethrum, permethrin, tetramethrin, phenothrin, cyfluthrin, bifenthrin Outdoor barrier: prallethrin, esfenvalerate, permethrin, beta-cyfluthrin, cyfluthrin, bifenthrin, malathion, carbaryl Outdoor broadcast: hydramethylnon, pyriproxyfen, beta-cyfluthrin, esfenvalerate, bifenthrin, cyfluthrin, malathion, carbaryl *Labels on insecticides should state “material may be used in the household” and should be registered by the EPA for that purpose. Household Insects – Homeowners ` CAUTION: All insecticides are toxic to some degree; therefore, care should be exercised in their use. -

The Toxic Effect of Permethrin and Cypermethrin on Engorged Ixodes
Annals of Agricultural and Environmental Medicine 2014, Vol 21, No 2, 259–262 ORIGINAL ARTICLE www.aaem.pl The toxic effect of permethrin and cypermethrin on engorged Ixodes ricinus females Alicja Buczek1, Katarzyna Bartosik1, Paweł Kuczyński1,2 1 Chair and Department of Biology and Parasitology, Medical University, Lublin, Poland 2 Chair and Department of Rehabilitation and Orthopaedics, Medical University, Lublin, Poland Buczek A, Bartosik K, Kuczyński P. The toxic effect of permethrin and cypermethrin on engorged Ixodes ricinus females. Ann Agric Environ Med. 2014; 21(2): 259–262. doi: 10.5604/1232-1966.1108587 Abstract Introduction. Ixodes ricinus tick is of great medical and veterinary importance and has a wide range of geographical distribution. The study presents the effect of permethrin (Per) and cypermethrin (CM) on engorged I. ricinus females. Materials and method. The effect of perythroids studied on engorged I. ricinus females was assessed on the basis of the pre-oviposition and oviposition period. Remote effects of Per and CM application were assessed by investigation of the length and course of embryonic development and larval hatching from eggs laid by pyrethroid-treated females. Per (Copex WP) was used at doses of 0.78125–25.0 µg/1 specimen, and CM (Kordon 10WP) was applied at 0.3125–10.0 µg/1 specimen. Immediately after the feeding period, I. ricinus females were sprayed with 20 µl of a pyrethroid solution and kept at 28 °C and 75%RH. Results. The experiments demonstrated that CM exerted a stronger toxic effect on I. ricinus females than Per. The lowest doses of CM doubled the length of the pre-oviposition period while its highest doses prolonged the period nearly three times compared with the control. -

Mosquito Control and Pollinator Health
“Though they spray for mosquitoes, bees find a way to visit.” Photo by Bev Veals, Kure Beach NC. Mosquito Control and Pollinator Health Protecting pollinators in the age of Zika and other emerging mosquito diseases by Nichelle Harriott In this piece, we explore how commonly used mosquito control pesticides and their application can potentially harm bees, n 2015, a beekeeper in Palo Alto, California, Randolph Tsien, butterflies, and other beneficial insects, ultimately affecting overall made local headlines after he reported the loss of hundreds of biodiversity. While we do not underestimate the threat from new and honey bees from his backyard hives following the local fogging for current mosquito-borne diseases, an ideal mosquito management I strategy adopts an integrated approach that emphasizes education, mosquitoes carrying West Nile virus. In addition to losing his honey bees, Mr. Tsien was concerned about the contamination of his honey, aggressive removal of breeding sites (such as standing water), which he once labeled ‘organic.’ Like Mr. Tsien, every year beekeepers larval control, monitoring, and surveillance. Alternative strategies, and concerned citizens worry about the impact of mosquito control including introducing mosquito-eating fish, encouraging predators, programs on honey bees and other pollinators. During the summer, such as bats, birds, dragonflies, and frogs, and using least-toxic mosquitoes become more active and the potential public health risks larvicides, like Bacillus thuringiensis (Bt), can be applied successfully associated with them begin to make national headlines. without endangering pollinators and other organisms. While mosquito-borne transmission of Zika virus has not been A note about wild native bees: While this article cites reported in the U.S., the virus has been found in travelers to the U.S. -

A Review of Insecticide Classes and Characteristics
Denver, CO | February 5 - 7, 2020 A Review of Insecticide Classes and Characteristics Whitney Cranshaw Colorado State University Common Types of Pesticides (Organisms Controlled) • Herbicides • Insecticides – Higher Plants – Insects • Algacides • Acaricides/ – Algae Miticides& Ticks • Fungicides • Molluscicides – Fungi – Slugs & Snails • Bactericides – Bacteria Classification of Insecticides Mode of Entry Classification of Insecticides Systemic or Not Systemic? Are they capable of moving within the plant? Distribution of C14 labeled Thiamethoxam™ 25WG after a foliar application to cucumber leaves 1 hour after application 8 hour after application 24 hour after application Slide Credit: N. Rechcigl Systemic insecticides applied to leaves Some systemic insecticide can move into plants when sprayed onto leaves. Some systemic insecticides can move into plant when applied to the roots. Most systemic insecticides will appear in highest concentration in the new growth Systemic insecticides applied to soil Systemic Insecticides • Capable of some translocation in plant • Range exists in ability to move in plant – Some limited to translaminar movement – Some broadly distribute in plant (usually to newer growth) • Systemic activity is limited to a small number of insecticides – Most neonicotinoids – Diamides (limited) – Abamectin (translaminar only) Systemic Insecticides • Capable of some translocation in plant • Range exists in ability to move in plant – Some limited to translaminar movement – Some broadly distribute in plant (usually to newer growth) • Systemic activity is limited to a small number of insecticides –Some organophophates –All neonicotinoids –Diamides (limited) –Avermectins (translaminar only) Translaminar movement – Insecticide can move through a leaf (but not necessarily to another leaf) Example: Foliar applications of abamectin (Avid) Essentially all systemic insecticide move primarily in the xylem of the plant. -

Managing Household Ant Pests Bastiaan M
B-6183 12-05 Managing Household Ant Pests Bastiaan M. Drees* n nature, ants are generally considered become queen ants in new colonies. They may to be beneficial insects. But when they choose indoor nesting sites if suitable ones are I invade a home, ants can be a nuisance. not available outdoors. When she finds a nesting To manage an ant infestation in the home, site, the queen loses her wings and begins to lay you must first identify the species. The next step eggs, which hatch into legless, grub-like larvae. is to learn about the biology of that species and The queen feeds the larvae as they develop determine where the colony might be nesting. through several stages, molting and growing Some species commonly nest indoors, while oth- between each stage. Larvae then form pupae and ers nest outside and enter a home just to look for soon emerge as adult ants. Once worker ants food. have developed, the queen no longer needs to To rid your home of ants, you must eliminate care for the brood. the colonies or nests. Some treatments, such When winged ants swarm in the home, it as insecticides sprayed on ant trails, kill only a is likely that their colony is located somewhere few foraging worker ants. They do not eliminate inside. Winged ants swarming outside, such as colonies. In fact, such treatments can sometimes around porch lights, should not be a concern. make the problem worse by causing a colony to To discourage them, turn off porch lights or use split into two or more separate colonies. -

Pyrethroids: How They Affect Human and Animal Health?
medicina Editorial Pyrethroids: How They Affect Human and Animal Health? Iga Hoły ´nska-Iwan 1,* and Karolina Szewczyk-Golec 2 1 Laboratory of Electrophysiology of Epithelial Tissue and Skin, Department of Pathobiochemistry and Clinical Chemistry, Faculty of Pharmacy, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, 87-100 Torun, Poland 2 Department of Medical Biology and Biochemistry, Faculty of Medicine, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, 87-100 Torun, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-525853598 Received: 21 October 2020; Accepted: 29 October 2020; Published: 30 October 2020 Abstract: Pyrethroids are pesticides commonly used in crop protection; in the forestry, wood, and textile industries; as well as in medicine and veterinary medicine to treat parasitic crustacean infestations. They have been found to be relatively safe for humans and animals. Pyrethroids are recommended for personal protection against malaria and virus Zika by the World Health Organization. Pyrethroids act on voltage-gated sodium channels, which cause an influx of sodium ions into the nerve cells and permanent depolarization. They also influence activities of enzymes, especially in nerve and liver cells. Contact of pyrethroids with the skin, digestive tract, and respiratory tract results in their penetration into the body. Due to the importance of the subject, a summary of the current state of knowledge on the toxic effects of pyrethroids was presented in the comprehensive review by Chrustek et al, published in journal Medicina. Particular attention was paid to nephrotoxic, hepatotoxic, cardiotoxic, immunotoxic, neurotoxic, and behavioral effects of pyrethroids on human and animal bodies. -

The Hazardous Substances and Waste Dangerous Goods Regulations
1 HAZARDOUS SUBSTANCES AND WASTE DANGEROUS GOODS E-10.2 REG 3 The Hazardous Substances and Waste Dangerous Goods Regulations being Chapter E-10.2 Reg 3 (effective April 1, 1989) as amended by Saskatchewan Regulations 25/92, 107/92, 28/94, 3/95 and 63/2000. NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. 2 HAZARDOUS SUBSTANCES AND E-10.2 REG 3 WASTE DANGEROUS GOODS Table of Contents 1 Title 11 Decision to grant approval INTERPRETATION 12 Approval not assignable, exception 2 Interpretation 13 Duties of operator, owner HAZARDOUS SUBSTANCES 14 Prohibition re storage in above-ground tanks 3 Designation of hazardous substances 15 Prohibition re storage in underground tanks 16 Prohibition re storage in certain containers or DESIGNATION AS HAZARDOUS WASTES stockpiles 3.1 Designation of waste dangerous goods 17 Decommissioning as hazardous wastes TRANSFERAL OF WASTE DANGEROUS GOODS CHARACTERIZATION OF SUBSTANCES 18 Transferal of waste dangerous goods 4 Characteristics of certain hazardous substances EXEMPTION FROM REQUIREMENTS Appendix A 5 General Exemptions INDUSTRIAL HAZARDOUS SUBSTANCES 6 Underground storage facilities Appendix B 7 Above-ground storage facilities ACUTE HAZARDOUS SUBSTANCES 8 Storage in small containers Appendix C APPROVAL TO STORE ENVIRONMENTAL PERSISTENT OR 9 Approval to store CHRONIC HAZARDOUS SUBSTANCES APPROVAL TO CONSTRUCT Appendix D 10 Approval to construct WASTE DANGEROUS GOODS 3 HAZARDOUS SUBSTANCES AND WASTE DANGEROUS GOODS E-10.2 REG 3 CHAPTER E-10.2 REG 3 The Environmental Management and Protection Act Title 1 These regulations may be cited as The Hazardous Substances and Waste Dangerous Goods Regulations. -

Methoxychlor
METHOXYCHLOR DRAFT RISK MANAGEMENT EVALUATION First Draft 9 April 2021 UNEP/POPS/POPRC.17/xxx Contents Executive Summary ............................................................................................................................................... 3 1. Introduction ...................................................................................................................................................... 4 1.1 Chemical identity of methoxychlor ......................................................................................................... 4 1.2 Production and uses ................................................................................................................................. 5 1.3 Conclusions of the POPs Review Committee regarding Annex E information .................................. 6 1.4 Data sources .............................................................................................................................................. 7 1.4.1 Overview of data submitted by Parties and observers............................................................ 7 1.4.2 Other data sources ..................................................................................................................... 7 1.5 Status of the chemical under International Conventions ..................................................................... 7 1.6 Any national or regional control actions taken ..................................................................................... 7 2. Summary of -

Lindane Lotion Over All Skin from the Neck Down
DOSAGE AND ADMINISTRATION Apply a thin layer of Lindane Lotion over all skin from the neck down. One ounce is sufficient for an average adult. Do not prescribe more than 2 ounces for larger adults. Apply only once. Wash off in 8 to 12 hours. Do not retreat. (See boxed WARNINGS.) Patients should be provided specific information on use of product. (See PRECAUTIONS: Information for Patients and the Medication Guide.) Patients should be instructed on proper use of Lindane Lotion, especially the amount to apply, how long to leave on and the need to avoid retreatment. Patients should be informed that itching occurs after the successful killing of scabies (lice) and continued itching is not necessarily an indication for retreatment with Lindane Lotion. A Lindane Lotion Medication Guide must be given to the patient each time Lindane Lotion is dispensed, as required by law. The Lindane Lotion Medication Guide is an important part of the risk management program for the patient. HOW SUPPLIED Lindane Lotion, USP 1% is supplied in patient-size 2 fl oz (60 mL) bottles. SHAKE WELL BEFORE USING Store at controlled room temperature, 15 ° – 30 °C (59 ° – 86 °F) [see USP]. REFERENCES 1. Feldmann, R.J. and Maibach, H.I., Toxicol. Applied. Pharmacol., 28:126, 1974. 2. Dale, W.E., Curly, A. and Cueto, C. Life Sci 5:47, 1966. 3. Ginsburg, C.M., et al., J. Pediatr. 91:6, 998–1000, 1977. 4. FDA AERS database search, January 2003 Rx Only Product No.: 8833 Manufactured By: Morton Grove Pharmaceuticals, Inc. Morton Grove, IL 60053 A50-8833-60 ISS.