Therapeutic Class Overview Scabicides and Pediculicides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidance on Identification of Alternatives to New Pops
Stockholm Convention on Persistent Organic Pollutants Guidance on identification of alternatives to new POPs Secretariat of the Stockholm Convention Concept of “Substitution” under the Stockholm Convention • The substitution is a strategy promoted by the Stockholm Convention to reach its objectives • Parties that are still producing or using the new POPs listed in Annex A, will need to search and identify alternatives to replace them • In the case of PFOS and for the exemptions for uses allowed by the Convention, these group of chemicals will be eventually prohibited and Parties are therefore encouraged to find alternatives to substitute them 2 Availability of alternatives • Currently, some countries have phased out the use of some of the new POPs, and there are feasible alternatives available to replace them Alternatives Chemical Name Use Ethoprop, oxamyl Pesticide to control banana root borer Cyfluthrin, Imidacloprid Pesticide to control tobacco wireworms Azadirachtin, bifenthrin, boric acid, carbaryl, Pesticide to control capsaicin, cypermethrin, cyfluthrin, ants and/or deltamethrin, diazinon, dichlorvos, cockroaches esfenvalerate, imidacloprid, lamda-cyhalothrin, Chlordecone malathion, permethrin, piperonyl butoxide, pyrethrins, pyriproxyfen, resmethrin, s- bioallerthrin, tetramethrin Bacillus thuringiensis, cultural practices such Pest management as crop rotation, intercropping, and trap cropping; barrier methods, such as screens, and bagging of fruit; use of traps such as pheromone and light traps to attract and kill insects. 3 -

Lindane – Product Discontinuation • the FDA Announced the Discontinuation of Lindane 1% Lotion and 1% Shampoo Due to Product Line Rationalization
Lindane – Product Discontinuation • The FDA announced the discontinuation of Lindane 1% lotion and 1% shampoo due to product line rationalization. — The discontinuation is not due to product quality, safety or efficacy concerns. • Lindane lotion is indicated for the treatment of scabies (infestations of Sarcoptes scabei) only in patients who cannot tolerate other approved therapies, or have failed treatment with other approved therapies. • Lindane shampoo is indicated for the treatment of head lice (infestations of Pediculus humanus capitis), crab lice (infestations of Pthirus pubis), and their ova only in patients who cannot tolerate other approved therapies, or have failed treatment with other approved therapies. • Other prescription products indicated to treat lice include Ulesfia® (benzyl alcohol) 5% lotion, Ovide® (malathion) 0.5% lotion, Natroba™ (spinosad) 0.9% topical suspension, and Sklice® (ivermectin) 0.5% lotion. — Over-the-counter products indicated to treat lice include various formulations of 1% permethrin (eg, Nix®), various formulations of piperonyl butoxide/pyrethrins (eg, CareOne® Lice), and other miscellaneous products such as Lycelle®. • Other prescription products indicated to treat scabies include Eurax® (crotamiton) 10% cream and 10% lotion and Elimite™ (permethrin) 5% cream. optumrx.com OptumRx® specializes in the delivery, clinical management and affordability of prescription medications and consumer health products. We are an Optum® company — a leading provider of integrated health services. Learn more at optum.com. All Optum® trademarks and logos are owned by Optum, Inc. All other brand or product names are trademarks or registered marks of their respective owners. This document contains information that is considered proprietary to OptumRx and should not be reproduced without the express written consent of OptumRx. -

Crotamiton, an Anti-Scabies Agent, Suppresses Histamine- and Chloroquine-Induced Itch Pathways in Sensory Neurons and Alleviates Scratching in Mice
Original Article Biomol Ther 28(6), 569-575 (2020) Crotamiton, an Anti-Scabies Agent, Suppresses Histamine- and Chloroquine-Induced Itch Pathways in Sensory Neurons and Alleviates Scratching in Mice Da-Som Choi1,2,†, Yeounjung Ji1,2,†, Yongwoo Jang3, Wook-Joo Lee1,2 and Won-Sik Shim1,2,* 1College of Pharmacy, Gachon University, Incheon 21936, 2Gachon Institute of Pharmaceutical Sciences, Incheon 21936, 3Department of Biomedical Engineering, Hanyang University, Seoul 04736, Republic of Korea Abstract Crotamiton is an anti-scabies drug, but it was recently found that crotamiton also suppresses non-scabietic itching in mice. How- ever, the underlying mechanism is largely unclear. Therefore, aim of the study is to investigate mechanisms of the anti-pruritic effect of crotamiton for non-scabietic itching. Histamine and chloroquine are used as non-scabietic pruritogens. The effect of crota- miton was identified using fluorometric intracellular calcium assays in HEK293T cells and primary cultured dorsal root ganglion (DRG) neurons. Further in vivo effect was evaluated by scratching behavior tests. Crotamiton strongly inhibited histamine-induced calcium influx in HEK293T cells, expressing both histamine receptor 1 (H1R) and transient receptor potential vanilloid 1 (TRPV1), as a model of histamine-induced itching. Similarly, it also blocked chloroquine-induced calcium influx in HEK293T cells, express- ing both Mas-related G-protein-coupled receptor A3 (MRGPRA3) and transient receptor potential A1 (TRPA1), as a model of histamine-independent itching. Furthermore, crotamiton also suppressed both histamine- and chloroquine-induced calcium influx in primary cultures of mouse DRG. Additionally, crotamiton strongly suppressed histamine- and chloroquine-induced scratching in mice. Overall, it was found that crotamiton has an anti-pruritic effect against non-scabietic itching by histamine and chloroquine. -

Scabies PAUL JOHNSTONE, Blenheim House, Leeds, United Kingdom MARK STRONG, University of Sheffield, Sheffield, United Kingdom
Clinical Evidence Handbook A Publication of BMJ Publishing Group Scabies PAUL JOHNSTONE, Blenheim House, Leeds, United Kingdom MARK STRONG, University of Sheffield, Sheffield, United Kingdom This is one in a series of Scabies is an infestation of the skin by the • Although tested in randomized chapters excerpted from mite Sarcoptes scabiei. In adults, the most controlled trials (RCTs), oral ivermectin is the Clinical Evidence Handbook, published by common sites of infestation are the fingers not presently licensed for the treatment of the BMJ Publishing Group, and the wrists, although it may manifest in scabies in most countries. It is only avail- London, U.K. The medical older persons as a diffuse truncal eruption. able on a named patient basis in the United information contained herein is the most accurate • Scabies is a very common public health Kingdom. available at the date of problem. In many resource-poor settings, it Topical lindane use has been restricted publication. More updated is an endemic problem; whereas in indus- or is not available in many parts of the and comprehensive infor- trialized countries, it is most common in world owing to the mounting evidence mation on this topic may be available in future print institutionalized communities. for serious adverse effects. We have not editions of the Clinical Evi- • Topical permethrin seems highly effec- included it in this review. However, it may dence Handbook, as well tive at increasing clinical cure of scabies be the most effective treatment that is as online at http://www. within 28 days. locally available in some countries. Harms clinicalevidence.bmj.com (subscription required). -

TITLE: Lindane and Other Treatments for Lice and Scabies: a Review of Clinical Effectiveness and Safety
TITLE: Lindane and Other Treatments for Lice and Scabies: A Review of Clinical Effectiveness and Safety DATE: 11 June 2010 CONTEXT AND POLICY ISSUES: Head lice infestation (Pediculosis capitis) affects millions of children and adults worldwide each year.1 Direct head-to-head contact is the most common mode of transmission.2 The highest prevalence of infestation occurs in school aged children aged three to eleven years, with girls being more commonly affected than boys.1,2 Although head lice are not generally associated with serious morbidity, they are responsible for significant social embarrassment and lost productivity in schools or offices.1 Scabies, an infestation of the skin by the mite Sarcoptes scabiei, represents a common public health concern particularly in overcrowded communities with a high prevalence of poverty.3 Scabies is transmitted by close-person contact and occasionally by clothing or linens.3 Complications include secondary bacterial infections and post-streptococcal glomerulonephritis.3 Topical products available in Canada for the treatment of head lice and scabies are presented in Appendix 1 and Appendix 2. Insecticidal agents such as permethrin and lindane have historically been considered the standard treatments for head lice and scabies.2,3 Toxicity is low following topical administration of permethrin due to minimal percutaneous absorption.4 However, several jurisdictions have banned lindane due to concerns of neurotoxicity and bone marrow suppression, as well as potential negative effects on the environment (contamination of waste water).5 Furthermore, widespread use of permethrin, pyrethrins/piperonyl butoxide, and lindane has led to resistance and higher rates of treatment failure.6 Resistance patterns and rates to these agents in Canada have not yet been studied.6 Due to concerns surrounding resistance and neurotoxicity, patients and caregivers have searched for alternative treatments. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 5/10/2013 Revision Date: 10/2/2018 Revision Number: G4 1. IDENTIFICATION Product identifier Product code: BE159 Product Name: BENZYL BENZOATE, USP Other means of identification Synonyms: Ascabin Ascabiol Benylate Benzyl alcohol benzoic ester Benzyl benzenecarboxylate Benzylets Benzyl phenylformate Benzoic acid, phenylmethyl ester Colebenz Novoscabin Peruscabin Scabanca Vanzoate Venzonate CAS #: 120-51-4 RTECS # DG4200000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Plasticizer for cellulose. Fixative. Dye carrier. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous according to the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Oral Category 4 Product code: BE159 Product name: BENZYL BENZOATE, 1 / 12 USP Label elements Warning Hazard statements Harmful if swallowed Hazards not otherwise classified (HNOC) Not Applicable Other hazards Toxic to aquatic life with long lasting effects May be harmful in contact with skin Precautionary Statements - Prevention Wash face, hands and any exposed skin thoroughly after handling Do not eat, drink or smoke when using this product Precautionary Statements - Response IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell Rinse mouth Precautionary Statements - Disposal Dispose of contents/container to an approved waste disposal plant 3. -
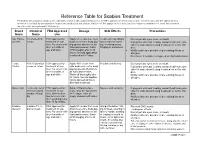
Reference Table for Scabies Treatment Permethrin and Crotamiton Products Are Commonly Used Over-The-Counter Products That Are FDA Approved Treatments for Scabies
Reference Table for Scabies Treatment Permethrin and crotamiton products are commonly used over-the-counter products that are FDA approved treatments for scabies. Invermectin is an FDA approved treat- ment that is available by prescription in both an oral medication and a lotion. Lindane is FDA approved for scabies but is no longer recommended as a first-line treatment for scabies due to its potential CNS toxicity. Brand Chemical FDA Approved Dosage Side Effects Precautions Name Name Use Nix, Elimite, 5% Permethrin FDA approved for Apply 5% cream from neck Treatment may initially Do not get into eyes, nose, or mouth Generic cream treatment of sca- down over entire body pay- make redness, swell- If pregnant or breast feeding, consult health care pro- bies. For use in chil- ing special attention to all ing, or itching worse. vider for instructions if using treatment on self or chil- dren 2 months of folds and creases. Wash Tingling or numbness. dren. age and older. off thoroughly after 8-14 Notify health care provider of pre-existing illness or hours. Second application allergies. is advised after 7 days. Do not use if sensitive to ragweed or chrysanthemums. Eurax, 10% Crotamiton FDA approved for Apply 10% cream from Irritation and itching Do not get into eyes, nose or mouth Crotan cream or lotion treatment of sca- chin down over entire body If pregnant or breast feeding, consult health care pro- bies. For use in chil- paying special attention to vider for instructions if using treatment on self or chil- dren 6 months of all folds and creases. -

Nebido 1000 Mg/4 Ml, Solution for Injection Testosterone Undecanoate
Bayer plc 400 South Oak Way, Reading, RG2 6AD Telephone: +44 (0) 118 206 3000 Medical information: [email protected]. www: http://www.bayer.co.uk Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end of the leaflet to establish if there have been any changes. If you have any doubts or queries about your medication, please contact your doctor or pharmacist. Package leaflet: Information for the user Nebido 1000 mg/4 ml, solution for injection Testosterone undecanoate Read all of this leaflet carefully before you are given this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Nebido is and what it is used for 2. What you need to know before you are given Nebido 3. How to use Nebido 4. Possible side effects 5. How to store Nebido 6. Contents of the pack and other information 1. What Nebido is and what it is used for Nebido contains testosterone, a male hormone, as the active ingredient. -

Injection Read This Medication Guide Before You Receive AVEED and Before Each Injection
MEDICATION GUIDE AVEED® (Uh-Veed) (testosterone undecanoate) injection Read this Medication Guide before you receive AVEED and before each injection. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment. What is the most important information I should know about AVEED? AVEED may cause serious side effects, including: • A serious lung problem. AVEED can cause a serious lung problem called a pulmonary oil microembolism (POME) reaction. POME is caused by tiny droplets of oil that have traveled to the lungs. Symptoms of a POME reaction may include: − cough or urge to cough − difficulty breathing − sweating − tightening of your throat − chest pain − dizziness − fainting • Serious allergic reactions (anaphylaxis). AVEED can cause a serious allergic reaction right after receiving the injection. Some of these allergic reactions may be life threatening. These reactions can happen after you receive your first dose of AVEED or may happen after receiving more than 1 dose. You may need emergency treatment in a hospital, especially if these symptoms get worse over the 24 hours after your AVEED injection. These side effects may happen during or right after each injection. To be sure that you are not having one of these reactions: • You need to stay in the doctor’s office, clinic, or hospital for 30 minutes after having your AVEED injection so that your doctor can watch you for symptoms of POME or a serious allergic reaction. • You can only get AVEED at your doctor’s office, clinic, or hospital. AVEED is only available through a restricted program called the AVEED Risk Evaluation and Mitigation Strategy (REMS) Program. -
Therapeutic Class Overview Scabicides and Pediculicides
Therapeutic Class Overview Scabicides and Pediculicides Therapeutic Class Overview/Summary: The agents indicated for the management of scabies and head lice are listed in Table 1. All of these agents are Food and Drug Administration (FDA)-approved for the treatment of head lice with the exception of crotamiton (Eurax®), which is only indicated to treat scabies.1-8 The scabicides and pediculicides have a neurotoxic effect on lice and scabies, resulting in periods of central nervous system hyperexcitation, ultimately resulting in paralysis and death of the parasite.1-8 Benzyl alcohol (Ulesfia®) is unique in that it disables the breathing structure of the lice, resulting in asphyxiation rather than neuroexcitation.1 The challenge of complete eradication of the infestation with a single treatment results from the fact that the neurotoxic insecticides rely on the nervous system to exert their effect; therefore, newborn larvae are not susceptible to these agents as they do not have intact nervous systems for several days after eggs are hatched. Pyrethrins (RID®) and permethrin (Nix®) are pediculicidal, but not ovicidal, and require nit combing and retreatment in 7 to 10 days. Resistance to pyrethrins and permethrin has been reported to be increasing in the United States. Benzyl alcohol is not ovicidal and also requires a second treatment, but resistance is unlikely due to its unique mechanism of action. Malathion is both pediculicidal and ovicidal, but must be applied for 8 to 12 hours, and is highly flammable. Lindane is neurotoxic and is not recommended as an initial treatment option.9 Ivermectin (Sklice®) and spinosad (Natroba®) are recently approved products that are pediculicidal but not ovicidal. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Household Insects – Homeowners ` CAUTION: All Insecticides Are Toxic to Some Degree; Therefore, Care Should Be Exercised in Their Use
Household Insects – Homeowners ` CAUTION: All insecticides are toxic to some degree; therefore, care should be exercised in their use. The manufacturer’s directions on the label in the use of the material must be followed explicitly. Insect Threats Insecticides and Treatment* Remarks Ants Feed on foods and Baits (active ingredient and Remove food and clean up the area. Place (several may damage product): bait where ants occur or congregate. May species) clothing; may also sodium tetraborate decahydrate use several different baits at the same time sting, causing severe (Amdro Kills Ants Liquid Bait, Terro to discover one that ants will consume. reaction to some Liquid Ant Baits); Care should be taken not to contaminate people. hydramethylnon (Amdro Kills Ants foodstuffs. Also treat nests in yard. Follow Bait Stations and Stakes); label. orthoboric acid (Terro Perimeter Ant Bait); fipronil (Combat Max Ant Killing Bait Stations and Gel); abamectin (Raid Max Double Control Ant Baits, Raid Ant Baits III); dinotefuran (Hot Shot Ultra Clear Roach & Ant Gel Bait, Hot Shot Ultra Liquid Ant Bait); spinosad (Ortho Home Defense Liquid Ant Bait); thiamethoxam (Raid Precision Placement Ant Bait Gel) Crack and crevices: Follow label. prallethrin, esfenvalerate, pyrethrins, pyrethrum, permethrin, tetra- methrin, phenothrin, beta-cyfluthrin, cyfluthrin Indoor space: prallethrin, esfenvalerate, pyrethrins, pyrethrum, permethrin, tetramethrin, phenothrin, cyfluthrin, bifenthrin Outdoor barrier: prallethrin, esfenvalerate, permethrin, beta-cyfluthrin, cyfluthrin, bifenthrin, malathion, carbaryl Outdoor broadcast: hydramethylnon, pyriproxyfen, beta-cyfluthrin, esfenvalerate, bifenthrin, cyfluthrin, malathion, carbaryl *Labels on insecticides should state “material may be used in the household” and should be registered by the EPA for that purpose. Household Insects – Homeowners ` CAUTION: All insecticides are toxic to some degree; therefore, care should be exercised in their use.