Nebido 1000 Mg/4 Ml, Solution for Injection Testosterone Undecanoate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scabies PAUL JOHNSTONE, Blenheim House, Leeds, United Kingdom MARK STRONG, University of Sheffield, Sheffield, United Kingdom
Clinical Evidence Handbook A Publication of BMJ Publishing Group Scabies PAUL JOHNSTONE, Blenheim House, Leeds, United Kingdom MARK STRONG, University of Sheffield, Sheffield, United Kingdom This is one in a series of Scabies is an infestation of the skin by the • Although tested in randomized chapters excerpted from mite Sarcoptes scabiei. In adults, the most controlled trials (RCTs), oral ivermectin is the Clinical Evidence Handbook, published by common sites of infestation are the fingers not presently licensed for the treatment of the BMJ Publishing Group, and the wrists, although it may manifest in scabies in most countries. It is only avail- London, U.K. The medical older persons as a diffuse truncal eruption. able on a named patient basis in the United information contained herein is the most accurate • Scabies is a very common public health Kingdom. available at the date of problem. In many resource-poor settings, it Topical lindane use has been restricted publication. More updated is an endemic problem; whereas in indus- or is not available in many parts of the and comprehensive infor- trialized countries, it is most common in world owing to the mounting evidence mation on this topic may be available in future print institutionalized communities. for serious adverse effects. We have not editions of the Clinical Evi- • Topical permethrin seems highly effec- included it in this review. However, it may dence Handbook, as well tive at increasing clinical cure of scabies be the most effective treatment that is as online at http://www. within 28 days. locally available in some countries. Harms clinicalevidence.bmj.com (subscription required). -

TITLE: Lindane and Other Treatments for Lice and Scabies: a Review of Clinical Effectiveness and Safety
TITLE: Lindane and Other Treatments for Lice and Scabies: A Review of Clinical Effectiveness and Safety DATE: 11 June 2010 CONTEXT AND POLICY ISSUES: Head lice infestation (Pediculosis capitis) affects millions of children and adults worldwide each year.1 Direct head-to-head contact is the most common mode of transmission.2 The highest prevalence of infestation occurs in school aged children aged three to eleven years, with girls being more commonly affected than boys.1,2 Although head lice are not generally associated with serious morbidity, they are responsible for significant social embarrassment and lost productivity in schools or offices.1 Scabies, an infestation of the skin by the mite Sarcoptes scabiei, represents a common public health concern particularly in overcrowded communities with a high prevalence of poverty.3 Scabies is transmitted by close-person contact and occasionally by clothing or linens.3 Complications include secondary bacterial infections and post-streptococcal glomerulonephritis.3 Topical products available in Canada for the treatment of head lice and scabies are presented in Appendix 1 and Appendix 2. Insecticidal agents such as permethrin and lindane have historically been considered the standard treatments for head lice and scabies.2,3 Toxicity is low following topical administration of permethrin due to minimal percutaneous absorption.4 However, several jurisdictions have banned lindane due to concerns of neurotoxicity and bone marrow suppression, as well as potential negative effects on the environment (contamination of waste water).5 Furthermore, widespread use of permethrin, pyrethrins/piperonyl butoxide, and lindane has led to resistance and higher rates of treatment failure.6 Resistance patterns and rates to these agents in Canada have not yet been studied.6 Due to concerns surrounding resistance and neurotoxicity, patients and caregivers have searched for alternative treatments. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 5/10/2013 Revision Date: 10/2/2018 Revision Number: G4 1. IDENTIFICATION Product identifier Product code: BE159 Product Name: BENZYL BENZOATE, USP Other means of identification Synonyms: Ascabin Ascabiol Benylate Benzyl alcohol benzoic ester Benzyl benzenecarboxylate Benzylets Benzyl phenylformate Benzoic acid, phenylmethyl ester Colebenz Novoscabin Peruscabin Scabanca Vanzoate Venzonate CAS #: 120-51-4 RTECS # DG4200000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Plasticizer for cellulose. Fixative. Dye carrier. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous according to the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Oral Category 4 Product code: BE159 Product name: BENZYL BENZOATE, 1 / 12 USP Label elements Warning Hazard statements Harmful if swallowed Hazards not otherwise classified (HNOC) Not Applicable Other hazards Toxic to aquatic life with long lasting effects May be harmful in contact with skin Precautionary Statements - Prevention Wash face, hands and any exposed skin thoroughly after handling Do not eat, drink or smoke when using this product Precautionary Statements - Response IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell Rinse mouth Precautionary Statements - Disposal Dispose of contents/container to an approved waste disposal plant 3. -
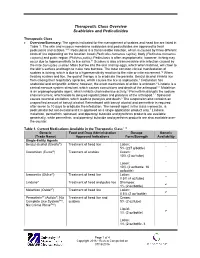
Therapeutic Class Overview Scabicides and Pediculicides
Therapeutic Class Overview Scabicides and Pediculicides Therapeutic Class Overview/Summary: The agents indicated for the management of scabies and head lice are listed in Table 1. The skin and mucous membrane scabicides and pediculicides are approved to treat pediculosis and scabies.1-10 Pediculosis is a transmissible infection, which is caused by three different kinds of lice depending on the location: head (Pediculus humanus capitis), body (Pediculus humanus corporis) and pubic region (Phthirus pubis). Pediculosis is often asymptomatic; however, itching may occur due to hypersensitivity to lice saliva.11 Scabies is also a transmissible skin infection caused by the mite Sarcoptes scabiei. Mites burrow into the skin and lay eggs, which when hatched, will crawl to the skin’s surface and begin to make new burrows. The most common clinical manifestation of scabies is itching, which is due to a hypersensitivity reaction to the mite or mite excrement.12 When treating scabies and lice, the goal of therapy is to eradicate the parasite. Benzyl alcohol inhibits lice from closing their respiratory spiracles, which causes the lice to asphyxiate.3 Crotamiton has scabicidal and antipruritic actions; however, the exact mechanism of action is unknown.4 Lindane is a central nervous system stimulant, which causes convulsions and death of the arthropod.1,2 Malathion is an organophosphate agent, which inhibits cholinesterase activity.5 Permethrin disrupts the sodium channel current, which leads to delayed repolarization and paralysis of the arthropod.1,2 Spinosad causes neuronal excitation, which leads to paralysis and death.6 The suspension also contains an unspecified amount of benzyl alcohol. -

Injection Read This Medication Guide Before You Receive AVEED and Before Each Injection
MEDICATION GUIDE AVEED® (Uh-Veed) (testosterone undecanoate) injection Read this Medication Guide before you receive AVEED and before each injection. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment. What is the most important information I should know about AVEED? AVEED may cause serious side effects, including: • A serious lung problem. AVEED can cause a serious lung problem called a pulmonary oil microembolism (POME) reaction. POME is caused by tiny droplets of oil that have traveled to the lungs. Symptoms of a POME reaction may include: − cough or urge to cough − difficulty breathing − sweating − tightening of your throat − chest pain − dizziness − fainting • Serious allergic reactions (anaphylaxis). AVEED can cause a serious allergic reaction right after receiving the injection. Some of these allergic reactions may be life threatening. These reactions can happen after you receive your first dose of AVEED or may happen after receiving more than 1 dose. You may need emergency treatment in a hospital, especially if these symptoms get worse over the 24 hours after your AVEED injection. These side effects may happen during or right after each injection. To be sure that you are not having one of these reactions: • You need to stay in the doctor’s office, clinic, or hospital for 30 minutes after having your AVEED injection so that your doctor can watch you for symptoms of POME or a serious allergic reaction. • You can only get AVEED at your doctor’s office, clinic, or hospital. AVEED is only available through a restricted program called the AVEED Risk Evaluation and Mitigation Strategy (REMS) Program. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Download Report
Discovery and Development of a Sumitomo Chemical Co., Ltd. Agricultural Chemicals Research Laboratory New Insecticide ‘amidoflumet Tatsuya MORI (Panduck®)’ with High Miticidal Noritada MATSUO Makoto HATAKOSHI Activity against House Dust Environmental Health Division Mites Yasuyori TANAKA Environmental Health Science Laboratory Keiko OSE Amidoflumet is a new trifluoromethanesulfonanilide compound with high house dust miticidal activity which was discovered by Sumitomo Chemical, and was registered in Japan in 2004. House dust mites and their prod- ucts are known to be major household allergens to children and the elderly, and they cause asthma and atopic dermatitis. Amidoflumet shows high lethal activity against common house dust mites. In particular amidoflumet has excellent activity against predatory cheyletid mites, which often cause biting injuries to humans. These effi- cacies and its excellent safety to mammals can provide us with an important tool for controlling various house dust mites. This paper describes the discovery story, miticidal efficacies in various formulations, a method of synthesis and safety evaluations of amidoflumet. This paper is translated from R&D Report, “SUMITOMO KAGAKU”, vol. 2007-II. Introduction tional miticides for indoor use, and we have discovered and developed amidoflumet (Panduck®), which is a Various types of mites inhabit the typical home envi- new compound that exhibits activity not seen in exist- ronment, but among these, house dust mites that feed ing miticides. Amidoflumet has a higher lethal efficacy on human dandruff and food residues are found in the for the house dust mites that inhabit households in largest numbers. Their corpses and feces cause aller- general than conventional agents and is faster acting, gies.1) Therefore, control of house dust mites is an and a salient feature is its high lethal efficacy against important problem in terms of measures for allergic cheyletid mites, which have been difficult to control disorders, which have been increasing in children and with the agents used up to now. -

Chemicals Used in Military Operations During the Vietnam War
Chemicals Used in Military Operations during the Vietnam War General use: Insecticide, DDT - Pyrethrum aerosol, G-1152, 12-oz, can.* Insecticide, Dichlorvos, 20% impregnated strips Insecticide, Lindane, 1% dusting powder, 2-oz. can** Insecticide, Pyrethrum, 0.6% aerosol, 12-oz. can Insecticide, Pyrethrum, 0.4% solution, 1-gal. can Repellent, Clothing and personal application, m 75% DEET, 6-oz. (aerosol can) Repellent, Clothing and personal application, m 75% DEET, 2-oz. (plastic bottle) Repellent, Clothing and personal application, m 75% DEET, ½-oz. (bottle, component of survival kit) Rodenticide, Anticoagulant, Ready mixed bait, 5-lb can Rodenticide, bait block, diphacin, 8-oz. block Supervision Required: Insecticide, Aluminum phosphide, tablets, can Insecticide, Aluminum phosphide, pellets, flask Insecticide, Baygon, 1% solution, 1-gal. can Insecticide, Baygon, 2% bait, 5-lb. bottle Insecticide, Carbaryl, 80% powder, 15-lb. pail Insecticide, Carbaryl-DDT, Micronized dust, 1-gram*** Insecticide, Carbaryl-DDT, Micronized dust, 5-gram*** Insecticide, Carbaryl-DDT, Micronized dust, 13-gram*** Insecticide, Chlordane, 72% emulsifiable concentrate, 5-gal. pail Insecticide, Chlordane, 5%-6% dust, 25-lb. pail Insecticide, Diazinon, 0.5% solution, 1-gal. can Insecticide, Diazinon, 48% emulsifiable concentrate, 1-gal. can Insecticide, Dieldrin, 15% emulsifiable concentrate, 5-gal. pail Insecticide, DDT, 25% emulsifiable concentrate, 5-gal. pail * For disinsectization of aircraft in compliance with Public Health Quarantine. ** For use in control of body lice. *** For disinsectization of aircraft in compliance with Agricultural Quarantine. 1 Insecticide, DDT, 75% wettable powder, 20-lb. pail Insecticide, Dichlorvos, 20% impregnated pellets, 30-lb. pail Insecticide, Dursban, 40.8% emulsifiable concentrate Insecticide, Lindane, 12% emulsifiable concentrate, 5-gal. -

Guidelines for Atcvet Classification 2012
Guidelines for ATCvet classification 2012 ISSN 1020-9891 ISBN 978-82-8082-479-0 Suggested citation: WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATCvet classification 2012. Oslo, 2012. © Copyright WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway. Use of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed. Guidelines for ATCvet classification 14th edition WHO Collaborating Centre for Drug Statistics Methodology Norwegian Institute of Public Health P.O.Box 4404 Nydalen N-0403 Oslo Norway Telephone: +47 21078160 Telefax: +47 21078146 E-mail: [email protected] Website: www.whocc.no Previous editions: 1992: Guidelines on ATCvet classification, 1st edition1) 1995: Guidelines on ATCvet classification, 2nd edition1) 1999: Guidelines on ATCvet classification, 3rd edition1) 2002: Guidelines for ATCvet classification, 4th edition2) 2003: Guidelines for ATCvet classification, 5th edition2) 2004: Guidelines for ATCvet classification, 6th edition2) 2005: Guidelines for ATCvet classification, 7th edition2) 2006: Guidelines for ATCvet classification, 8th edition2) 2007: Guidelines for ATCvet classification, 9th edition2) 2008: Guidelines for ATCvet classification, 10th edition2) 2009: Guidelines for ATCvet classification, 11th edition2) 2010: Guidelines for ATCvet classification, 12th edition2) 2011: Guidelines for ATCvet classification, 13th edition2) 1) Published by the Nordic Council on Medicines 2) Published by the WHO Collaborating Centre for Drug Statistics Methodology Preface The Anatomical Therapeutic Chemical classification system for veterinary medicinal products, ATCvet, has been developed by the Nordic Council on Medicines (NLN) in collaboration with the NLN’s ATCvet working group, consisting of experts from the Nordic countries. -

Clinical Trial Results Summary Study IP157-003 1
Clinical Trial Results Summary Study IP157-003 Study Number: IP157-003 Title of Study: Phase I, Double-Blind Study to Evaluate the Allergic Potential of NEBIDO® and Formulation Components in Patients Who Have Exhibited Anaphylactic-Like Reactions Following Intramuscular Injection of NEBIDO® Principal Investigator: Torsten Zuberbier, MD Study center(s): Allergy Centre Charité, Charité-Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany Publications (reference): None Studied Period (years): Phase of Development: 1 Date first patient enrolled: 13-Sep-2010 Date last patient completed: 15-Sep-2010 Objectives: The overall objective of the study was to evaluate, using skin prick testing and intramuscular (IM) injection, the possible nature and etiology of anaphylactic-like reactions to NEBIDO and its formulation components (castor oil and benzyl benzoate) in patients who were reported to have experienced spontaneous anaphylactic-like adverse events (AEs) following injection of NEBIDO. Methodology: A diagnostic workup was to be performed in this Phase 1 double-blind, single-center study in 4 patients identified from postmarketing safety surveillance with reported anaphylactic-like reactions to NEBIDO following IM injection. The NEBIDO preparation as well as each ingredient in the preparation was to be tested for its allergic potential in these patients. The study included: 1. A telephone interview for screening purposes by the investigator at time of recruitment to assess detailed patient history, to ascertain special needs during the stay of the patients (eg, special meals). The telephone interview included: • Medical history, including current medications; • Medicinal, food and environmental allergies; and • Past and present pulmonary history 2. A telephone call directly prior to travel to make sure patients had no acute infectious disease (eg, common cold with bronchitis); and 3. -

Stockholm Convention on Persistent Organic Pollutants
UNITED NATIONS SC UNEP/POPS/COP.6/INF/4/Rev.1 Distr.: General 24 April 2013 Stockholm Convention English only on Persistent Organic Pollutants Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants Sixth meeting Geneva, 28 April–10 May 2013 Item 5 (a) (ii) of the provisional agenda∗ Matters related to the implementation of the Convention: measures to reduce or eliminate releases from intentional production and use: exemptions Report on a study of health sector information sources on the availability of lindane as a pharmaceutical and its alternatives as a treatment for head lice and scabies Note by the Secretariat As referred to in document UNEP/POPS/COP.6/5 on the register of specific exemptions and the register of acceptable purposes, the annex to the present note sets out the report of a study, undertaken by the Secretariat, in cooperation with the World Health Organization, of health sector information sources on the availability of lindane as a pharmaceutical and its alternatives as a treatment for head lice and scabies. The annex has not been formally edited. ∗ UNEP/POPS/COP.6/1. K1351460 260413 UNEP/POPS/COP.6/INF/4/Rev.1 Annex SECRETARIAT OF THE STOCKHOLM CONVENTION Pharmaceutical uses of lindane as a treatment for scabies and head lice 2 UNEP/POPS/COP.6/INF/4/Rev.1 Acknowledgements The Secretariat of the Stockholm Convention acknowledges the collaboration and support extended by the Department of Essential Medicines and Healthcare Products of the World Health Organization in planning and collecting information for the development of this report. -

Q What Is the Most Effective Treatment for Scabies?
Evidence-based answers from the Family Physicians Inquiries Network CLINICAL INQUIRIES ONLINE EXCLUSIVE Jonathon J. Campbell, MD; Christopher P. Q What is the most effective Paulson, MD, FAAFP United States Air Force Eglin Family Medicine Residency, treatment for scabies? Fla Joan Nashelsky, MLS EVIDENCE-BASED ANSWER University of Iowa, Iowa City Topical permethrin is the most Although not as effective as topical DEPUTY EDITOR A effective treatment for classic sca- permethrin, oral ivermectin is an effective Rick Guthmann, MD, MPH bies (strength of recommendation [SOR]: treatment compared with placebo (SOR: B, Advocate Illinois Masonic A, meta-analyses with consistent results). a single small randomized trial). Family Medicine Residency, Topical lindane and crotamiton are in- Oral ivermectin may reduce the preva- Chicago ferior to permethrin but appear equivalent lence of scabies at one year in populations to each other and benzyl benzoate, sulfur, with endemic disease more than topical and natural synergized pyrethrins (SOR: B, permethrin (SOR: B, a single randomized limited randomized trials). trial). Evidence summary tients) showed no difference in treatment A 2007 Cochrane review on scabies treatment failures, but the trials were small and lacked identified 11 trials that evaluated permethrin sufficient statistical power. for treating scabies.1 In 2 trials, 140 patients Four additional studies included in were randomized to receive either 200 mcg/kg the review compared crotamiton with lin- of oral ivermectin or overnight application of dane (100 patients), lindane with sulfur 5% topical permethrin. Topical permethrin (68 patients), benzyl benzoate with sulfur was superior to oral ivermectin with failure (158 patients), and benzyl benzoate with rates at 2 weeks of 8% and 39%, respectively natural synergized pyrethrins (240 patients).