Chapter 2 Aspects of Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction What Is a Polymer Capacitor?
ECAS series (polymer-type aluminum electrolytic capacitor) No. C2T2CPS-063 Introduction If you take a look at the main board of an electronic device such as a personal computer, you’re likely to see some of the six types of capacitors shown below (Fig. 1). Common types of capacitors include tantalum electrolytic capacitors (MnO2 type and polymer type), aluminum electrolytic capacitors (electrolyte can type, polymer can type, and chip type), and MLCC. Figure 1. Main Types of Capacitors What Is a Polymer Capacitor? There are many other types of capacitors, such as film capacitors and niobium capacitors, but here we will describe polymer capacitors, a type of capacitor produced by Murata among others. In both tantalum electrolytic capacitors and aluminum electrolytic capacitors, a polymer capacitor is a type of electrolytic capacitor in which a conductive polymer is used as the cathode. In a polymer-type aluminum electrolytic capacitor, the anode is made of aluminum foil and the cathode is made of a conductive polymer. In a polymer-type tantalum electrolytic capacitor, the anode is made of the metal tantalum and the cathode is made of a conductive polymer. Figure 2 shows an example of this structure. Figure 2. Example of Structure of Conductive Polymer Aluminum Capacitor In conventional electrolytic capacitors, an electrolyte (electrolytic solution) or manganese dioxide (MnO2) was used as the cathode. Using a conductive polymer instead provides many advantages, making it possible to achieve a lower equivalent series resistance (ESR), more stable thermal characteristics, improved safety, and longer service life. As can be seen in Fig. 1, polymer capacitors have lower ESR than conventional electrolytic Copyright © muRata Manufacturing Co., Ltd. -

Capacitors and Inductors
DC Principles Study Unit Capacitors and Inductors By Robert Cecci In this text, you’ll learn about how capacitors and inductors operate in DC circuits. As an industrial electrician or elec- tronics technician, you’ll be likely to encounter capacitors and inductors in your everyday work. Capacitors and induc- tors are used in many types of industrial power supplies, Preview Preview motor drive systems, and on most industrial electronics printed circuit boards. When you complete this study unit, you’ll be able to • Explain how a capacitor holds a charge • Describe common types of capacitors • Identify capacitor ratings • Calculate the total capacitance of a circuit containing capacitors connected in series or in parallel • Calculate the time constant of a resistance-capacitance (RC) circuit • Explain how inductors are constructed and describe their rating system • Describe how an inductor can regulate the flow of cur- rent in a DC circuit • Calculate the total inductance of a circuit containing inductors connected in series or parallel • Calculate the time constant of a resistance-inductance (RL) circuit Electronics Workbench is a registered trademark, property of Interactive Image Technologies Ltd. and used with permission. You’ll see the symbol shown above at several locations throughout this study unit. This symbol is the logo of Electronics Workbench, a computer-simulated electronics laboratory. The appearance of this symbol in the text mar- gin signals that there’s an Electronics Workbench lab experiment associated with that section of the text. If your program includes Elec tronics Workbench as a part of your iii learning experience, you’ll receive an experiment lab book that describes your Electronics Workbench assignments. -
100“ 168\A166\1::: 1:1 162 { 164C 1
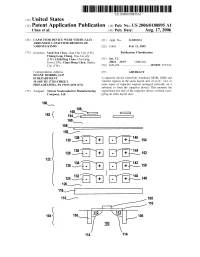
US 20060180895Al (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0180895 A1 Chen et al. (43) Pub. Date: Aug. 17, 2006 (54) CAPACITOR DEVICE WITH VERTICALLY (21) Appl. No.: 11/055,933 ARRANGED CAPACITOR REGIONS OF VARIOUS KINDS (22) Filed: Feb. 11, 2005 (75) Inventors: Yueh-You Chen, Hsin-Chu City (TW); Publication Classi?cation Chung-Long Chang, Dou-Liu city (TW); Chih-Ping Chao, Chu-Dong (51) Int. Cl. Town (TW); Chun-Hong Chen, Jhubei H01L 29/93 (2006.01) City (TW) (52) U.S. Cl. .......................................... .. 257/595; 257/E2l Correspondence Address: (57) ABSTRACT DUANE MORRIS, LLP IP DEPARTMENT A capacitor device selectively combines MOM, MIM and 30 SOUTH 17TH STREET varactor regions in the same layout area of an IC. TWo or PHILADELPHIA, PA 19103-4196 (US) more types of capacitor regions arranged vertically on a substrate to form the capacitor device. This increase the (73) Assignee: Taiwan Semiconductor Manufacturing capacitance per unit of the capacitor device, Without occu Company, Ltd. pying an extra layout area. 100“ 168\A166\1::: 1:1 162 { 164C 1:: . 160 158, F I /_\~ ’/'_‘\ 130 Bi- - + - +--E6_,154 /\_ A/A\ 128 i. - + - +--?, 152 122 ' 134 . ,/—\ 142 126 \_/—'—~ . + .. +_-\_, 150 /\'_ ‘/_\ 4 124 i. + - +--—2, 148 120% 118/1- ] 116f 108 112 112 104 10s \ p — /_/ 106 _ m _ 114 l_/114 110 Patent Application Publication Aug. 17, 2006 Sheet 2 0f 2 US 2006/0180895 A1 200“ - + - + + - + - - + - + + - + - FIG. 2 300% 316% + ' + 314“ 312% + + 310v“ ' 308 “w - + - + 306\___ 304“ + + FIG. 3 US 2006/0180895 A1 Aug. -

Capacitor & Capacitance
CAPACITOR & CAPACITANCE - TYPES Capacitor types Listed by di-electric material. A 12 pF 20 kV fixed vacuum capacitor Vacuum : Two metal, usually copper, electrodes are separated by a vacuum. The insulating envelope is usually glass or ceramic. Typically of low capacitance - 10 - 1000 pF and high voltage, up to tens of kilovolts, they are most often used in radio transmitters and other high voltage power devices. Both fixed and variable types are available. Vacuum variable capacitors can have a minimum to maximum capacitance ratio of up to 100, allowing any tuned circuit to cover a full decade of frequency. Vacuum is the most perfect of dielectrics with a zero loss tangent. This allows very high powers to be transmitted without significant loss and consequent heating. Air : Air dielectric capacitors consist of metal plates separated by an air gap. The metal plates, of which there may be many interleaved, are most often made of aluminium or silver-plated brass. Nearly all air dielectric capacitors are variable and are used in radio tuning circuits. Metallized plastic film: Made from high quality polymer film (usually polycarbonate, polystyrene, polypropylene, polyester (Mylar), and for high quality capacitors polysulfone), and metal foil or a layer of metal deposited on surface. They have good quality and stability, and are suitable for timer circuits. Suitable for high frequencies. Mica: Similar to metal film. Often high voltage. Suitable for high frequencies. Expensive. Excellent tolerance. Paper: Used for relatively high voltages. Now obsolete. Glass: Used for high voltages. Expensive. Stable temperature coefficient in a wide range of temperatures. Ceramic: Chips of alternating layers of metal and ceramic. -

3. PPE Materials Components and Devices 2019 USPAS
Pulsed Power Engineering: Materials & Passive Components and Devices U.S. Particle Accelerator School University of New Mexico Craig Burkhart & Mark Kemp SLAC National Accelerator Laboratory June 24-28, 2019 Materials & Passive Components and Devices Used in Pulsed Power Engineering - Materials • Conductors • Insulators • Magnetic material - Passive components and devices • Resistors • Capacitors • Inductors • Transformers • Transmission lines • Loads - Klystrons - Beam kickers Jun. 24-28, 2019 USPAS Pulsed Power Engineering C. Burkhart & M. Kemp 2 Materials - Generally encounter three types of materials in pulsed power work • Conductors - Wires & cable - Buss bars - Shielding - Resistors • Insulators - Cables and bushing - Standoffs - Capacitors • Magnetic - Inductors, transformers, and magnetic switches - Ferrite and tape-wound Jun. 24-28, 2019 USPAS Pulsed Power Engineering C. Burkhart & M. Kemp 3 Calculating Resistance - At low frequency, resistance (R) determined by: • R = ρℓ/A (ohm) - Material resistivity, ρ (Ω•cm) - Conductor length, ℓ (cm) - Conductor cross-sectional area, A (cm2) - At high frequency, effective conductor area decreased by “skin effect” • Conducted current produces magnetic field • Magnetic field induces eddy currents in conductor which oppose/cancel B • Eddy currents decay due to material resistance, allow conducted current/magnetic field to penetrate material • Skin depth, δ, is the effective conducted current penetration (B = Bapplied/e) • δ = (2ρ/μω)½ (meters) for a current of a fixed frequency ω=2πf, or δ ≈ (2tρ/μ)½ (meters) for a pulsed current of duration t (sec) - Material resistivity, ρ (Ω•m) - Material permeability, μ (H/m) ½ ½ • δ = (6.6/f )[(ρ/ρc)/(μ/μo)] (cm) -8 - Normalized resistivity, (ρ/ρc) , copper resistivity, ρc = 1.7 X 10 (Ω•m) -7 - Relative permeability, μr =(μ/μo), permeability of free space, μo = 4π X 10 (H/m) • Litz wire is woven to minimize skin effects Jun. -

MT-101: Decoupling Techniques
MT-101 TUTORIAL Decoupling Techniques WHAT IS PROPER DECOUPLING AND WHY IS IT NECESSARY? Most ICs suffer performance degradation of some type if there is ripple and/or noise on the power supply pins. A digital IC will incur a reduction in its noise margin and a possible increase in clock jitter. For high performance digital ICs, such as microprocessors and FPGAs, the specified tolerance on the supply (±5%, for example) includes the sum of the dc error, ripple, and noise. The digital device will meet specifications if this voltage remains within the tolerance. The traditional way to specify the sensitivity of an analog IC to power supply variations is the power supply rejection ratio (PSRR). For an amplifier, PSRR is the ratio of the change in output voltage to the change in power supply voltage, expressed as a ratio (PSRR) or in dB (PSR). PSRR can be referred to the output (RTO) or referred to the input (RTI). The RTI value is equal to the RTO value divided by the gain of the amplifier. Figure 1 shows how the PSR of a typical high performance amplifier (AD8099) degrades with frequency at approximately 6 dB/octave (20 dB/decade). Curves are shown for both the positive and negative supply. Although 90 dB at dc, the PSR drops rapidly at higher frequencies where more and more unwanted energy on the power line will couple to the output directly. Therefore, it is necessary to keep this high frequency energy from entering the chip in the first place. This is generally done with a combination of electrolytic capacitors (for low frequency decoupling), ceramic capacitors (for high frequency decoupling), and possibly ferrite beads. -

Wei Thesis.Pdf
UNIVERSITY OF CALIFORNIA Santa Barbara Wide Bandwidth Power Heterojunction Bipolar Transistors and Amplifiers A Dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Electrical and Computer Engineering by Yun Wei Committee in charge: Professor Mark Rodwell, Chair Professor Steve Long Professor Umesh Mishra Professor Robert York March, 2003 The dissertation of Yun Wei is approved. _____________________________________________ Professor Steve Long _____________________________________________ Professor Umesh Mishra _____________________________________________ Professor Robert York _____________________________________________ Professor Mark Rodwell, Committee Chair November, 2002 ii Wide Bandwidth Power Heterojunction Bipolar Transistors and Amplifiers Copyright © 2003 by Yun Wei iii To my wife and daughter. iv ACKNOWLEDGEMENTS I would like to say that I feel lucky of being a student of Prof. Rodwell. I am always indebted to him for his advice and assistance during my graduate school career. I have learned not only the knowledge, but also gained a lot from his enthusiasm for scientific research and willingness to help others. I would also like to thank my committee members Prof. Umesh Mishra, Prof. Stephen Long and Prof. Robert York for their patience, encouragement and support. It was my greatest pleasure of working with my colleagues, who gave me both helps and friendliness. Thanks to Dino, James, Shri, Tomas and PK, who gave me the first laboratory training. Special thanks to Paidi, Miguel and Dennis; this thesis would not have been possible without their contributions. Also thanks to Sangmin, Zach, Shouxuan, Mattias, and Navin for their suggestions and support during this work. The excellent support received from Jack Whaley, Mike Anzlowar, Bob Hill, and Neil Baker led to the successful fabrication of the devices and MMICs described in this work. -

General Technical Information Vishay Roederstein Film Capacitors
General Technical Information www.vishay.com Vishay Roederstein Film Capacitors FILM CAPACITORS RFI suppression capacitors are the most effective way to Plastic film capacitors are generally subdivided into film/foil reduce RF energy interference. As its impedance decrease capacitors and metalized film capacitors. with frequency, it acts as a short-circuit for high-frequencies between the mains terminals and/or between the mains FILM / FOIL CAPACITORS terminals and the ground. Film / foil capacitors basically consist of two metal foil Capacitors for applications between the mains terminals are electrodes that are separated by an insulating plastic film called X Class capacitors. Capacitors for applications also called dielectric. The terminals are connected to the between the terminals and the ground are called Y Class end-faces of the electrodes by means of welding or capacitors. soldering. X-Capacitors Main features: For the suppression of symmetrical interference voltage. High insulation resistance, excellent current carrying and Capacitors with unlimited capacitance for use where their pulse handling capability and a good capacitance stability. failure will not lead to the danger of electrical shock on human beings and animals. The capacitor must present a METALIZED FILM CAPACITORS safe end of life behavior. The electrodes of metalized film capacitors consist of an Y-Capacitors extremely thin metal layer (0.02 μm to 0.1 μm) that is vacuum Capacitors for suppression of asymmetrical interference deposited either onto the dielectric film or onto a carrier film. voltage, and are located between a live wire and a metal The opposing and extended metalized film layers of the case which may be touched. -

2. Capacitors Contents
2. Capacitors Contents 1 Capacitor 1 1.1 History ................................................. 2 1.2 Theory of operation .......................................... 2 1.2.1 Overview ........................................... 3 1.2.2 Hydraulic analogy ....................................... 3 1.2.3 Energy of electric field .................................... 4 1.2.4 Current–voltage relation ................................... 4 1.2.5 DC circuits .......................................... 4 1.2.6 AC circuits .......................................... 5 1.2.7 Laplace circuit analysis (s-domain) .............................. 5 1.2.8 Parallel-plate model ...................................... 5 1.2.9 Networks ........................................... 6 1.3 Non-ideal behavior .......................................... 7 1.3.1 Breakdown voltage ...................................... 7 1.3.2 Equivalent circuit ....................................... 7 1.3.3 Q factor ............................................ 8 1.3.4 Ripple current ......................................... 8 1.3.5 Capacitance instability .................................... 8 1.3.6 Current and voltage reversal ................................. 9 1.3.7 Dielectric absorption ..................................... 9 1.3.8 Leakage ............................................ 9 1.3.9 Electrolytic failure from disuse ................................ 9 1.4 Capacitor types ............................................ 9 1.4.1 Dielectric materials ..................................... -

Aluminum Electrolytic Capacitors
Aluminum Electrolytic Capacitors Surface Mount Type FK series V type High temperature Lead-Free reflow (suffix : A✽) Features ● Endurance:105 ℃ 2000 h ● Low impedance (40 % to 60 % less than FC series) ● Miniaturized (30 % to 50 % less than FC series) ● Vibration-proof product (30G guaranteed) is available upon request(φ6.3 ≦) ● AEC-Q200 compliant ● RoHS compliant Specifications Category temp. range –55 ℃ to +105 ℃ Rated voltage range 6.3 V to 35 V Capacitance range 4.7 μF to 1500 μF Capacitance tolerance ±20 % (120 Hz / +20 ℃) Leakage current I ≦ 0.01 CV or 3 (μA) After 2 minutes (Whichever is greater) Dissipation factor (tan δ) Please see the attached characteristics list Rated voltage (V) 6.3 10 16 25 35 Characteristics Z (–25 ℃) / Z (+20 ℃) 2 2 2 2 2 (Impedance ratio at 120 Hz) at low temperature Z (–40 ℃) / Z (+20 ℃) 3 3 3 3 3 Z (–55 ℃) / Z (+20 ℃) 4 4 4 3 3 After applying rated working voltage for 2000 hours at +105 ℃ ± 2 ℃ and then being stabilized at +20 ℃, capacitors shall meet the following limits. Endurance Capacitance change Within ±30 % of the initial value Dissipation factor(tan δ) ≦ 200 % of the initial limit Leakage current Within the initial limit After storage for 1000 hours at +105 ℃ ± 2 ℃ with no voltage applied and then being Shelf life stabilized at +20 ℃, capacitors shall meet the limits specified in endurance. (With voltage treatment) After reflow soldering and then being stabilized at +20 ℃, capacitors shall meet the Resistance to following limits. Capacitance change Within ±10 % of the initial value soldering heat Dissipation factor(tan δ) Within the initial limit Leakage current Within the initial limit Frequency correction factor for ripple current Freq.(Hz) Cap.(μF) 120 1 k 10 k 100 k to 4.7 to 470 0.65 0.85 0.95 1.00 680 to 1500 0.70 0.90 0.95 1.00 Marking Dimensions 0.3 max. -

AN203: 8-Bit MCU Printed Circuit Board Design Notes
AN203: 8-bit MCU Printed Circuit Board Design Notes The tips and techniques included in this application note will help to ensure successful printed circuit board (PCB) design. KEY POINTS Problems in design can result in noisy and distorted analog measurements, error-prone • Power and ground circuit design tips. digital communications, latch-up problems with port pins, excessive electromagnetic in- • Analog and digital signal design terference (EMI), and other undesirable system behavior. recommendations with special tips for traces that require particular attention, The methods presented in this application note should be taken as suggestions which such as clock, voltage reference, and the reset signal traces. provide a good starting point in the design and layout of a PCB. It should be noted that one design rule does not necessarily fit all designs. It is highly recommended that pro- • Special requirements for designing totype PCBs be manufactured to test designs. For further information on any of the top- systems in electrically noisy environments. ics discussed in this application note, please read the works cited in 11. References. • Techniques for optimal design using multilayer boards. The information in this application note applies to all 8-bit MCUs (EFM8 and C8051). • A design checklist. PCB power connection bulk decoupling and bypass circuit conductor: traces or capacitors power planes Voltage Regulator IC IC local decoupling and bypass capacitors silabs.com | Smart. Connected. Energy-friendly. Rev. 0.3 AN203: 8-bit MCU Printed Circuit Board Design Notes Power and Ground 1. Power and Ground All embedded system designs have a power supply and ground circuit loop that is shared by components on the PCB. -

Selecting and Applying Aluminum Electrolytic Capacitors for Inverter Applications
Selecting and Applying Aluminum Electrolytic Capacitors for Inverter Applications Sam G. Parler, Jr. Cornell Dubilier Abstract— Aluminum electrolytic capacitors are widely used in all types of inverter power systems, from variable-speed drives to welders to UPS units. This paper discusses the considerations involved in selecting the right type of aluminum electro- lytic bus capacitors for such power systems. The relationship among temperature, voltage, and ripple ratings and how these parameters affect the capacitor life are discussed. Examples of how to use Cornell Dubilier’s web-based life-modeling java applets are covered. Introduction a knowledge of all aspects of the application environ- ment, from mechanical to thermal to electrical. The goal One of the main application classes of aluminum elec- of this paper is to assist you with selecting the right trolytic capacitors is input capacitors for power invert- capacitor for the design at hand. ers. The aluminum electrolytic capacitor provides a Capacitor ripple current waveform considerations unique value in high energy storage and low device impedance. How you go about selecting the right ca- Inverters generally use an input capacitor between a pacitor or capacitors, however, is not a trivial matter. rectified line input stage and a switched or resonant Selecting the right capacitor for an application requires converter stage. See Figure 1 below. There is also usu- (a) (b) Current Spectrum 2.5 2 1.5 Ck 1 0.5 0 1 10 100 1000 10000 k d=10% d=5% d=2.5% (d) (c) Figure 1: Inverter schematics. Clockwise: (a) block diagram of a typical DC power supply featuring an inverter stage, (b) motor drive inverter schematic shows the rectification stage, (c) typical inverter capacitor current waveforms, (d) relative capacitor ripple current frequency spectrum for various charge current duties (d=Ic/IL ).