From the Western Cape
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Asphodelus Fistulosus (Asphodelaceae, Asphodeloideae), a New Naturalised Alien Species from the West Coast of South Africa ⁎ J.S
Available online at www.sciencedirect.com South African Journal of Botany 79 (2012) 48–50 www.elsevier.com/locate/sajb Research note Asphodelus fistulosus (Asphodelaceae, Asphodeloideae), a new naturalised alien species from the West Coast of South Africa ⁎ J.S. Boatwright Compton Herbarium, South African National Biodiversity Institute, Private Bag X7, Claremont 7735, South Africa Department of Botany and Plant Biotechnology, University of Johannesburg, P.O. Box 524, Auckland Park 2006, Johannesburg, South Africa Received 4 November 2011; received in revised form 18 November 2011; accepted 21 November 2011 Abstract Asphodelus fistulosus or onionweed is recorded in South Africa for the first time and is the first record of an invasive member of the Asphodelaceae in the country. Only two populations of this plant have been observed, both along disturbed roadsides on the West Coast of South Africa. The extent and invasive potential of this infestation in the country is still limited but the species is known to be an aggressive invader in other parts of the world. © 2011 SAAB. Published by Elsevier B.V. All rights reserved. Keywords: Asphodelaceae; Asphodelus; Invasive species 1. Introduction flowers (Patterson, 1996). This paper reports on the presence of this species in South Africa. A population of A. fistulosus was The genus Asphodelus L. comprises 16 species distributed in first observed in the early 1990's by Drs John Manning and Eurasia and the Mediterranean (Días Lifante and Valdés, 1996). Peter Goldblatt during field work for their Wild Flower Guide It is superficially similar to the largely southern African to the West Coast (Manning and Goldblatt, 1996). -

Spider Plant Chlorophytum Comosum
Spider Plant Chlorophytum comosum Popular, durable, exotic—Spider Plant is an easy houseplant to grow and enjoy. Spider or Airplane Plants have either one of three leaf color patterns: solid green leaves, green edges with a white variegated stripe down the center of the leaf blade or leaves with white edges and a green stripe down the center. Basics: This easy to grow plant is more tolerant of extreme conditions than other houseplants, but it still has its climate preferences. Spider Plant thrives in cool to average home temperatures and partially dry to dry soil. Bright indirect light is best. Direct sunlight may cause leaf tip burn. Fertilizer may be applied monthly from March through September. A professional potting media containing sphagnum peat moss and little to no perlite is best. Special Care: Spider Plants store food reserves in adapted structures on the plants roots. These “swollen roots can actually push the plant up and out or even break the pot. Avoid over fertilizing to minimize this growth characteristic. Spider Plants are easy to propagate. Simply cut off one of the “spiders” or plantlets and place in a pot. You may need to pin it down to the surface of the potting media to hold it in place until the roots grow and anchor it. A paper clip bent into an elongated U shape does the trick. Spider Plants are photoperiodic, that is they respond to long uninterrupted periods of darkness (short day, long nights) by initiating flowering. Production of “spiders” follows flowering. This daylength occurs naturally in the fall of each year. -

<I>Chlorophytum Burundiense</I> (Asparagaceae), a New Species
Plant Ecology and Evolution 144 (2): 233–236, 2011 doi:10.5091/plecevo.2011.609 SHORT COMMUNICATION Chlorophytum burundiense (Asparagaceae), a new species from Burundi and Tanzania Pierre Meerts Herbarium et Bibliothèque de botanique africaine, Université Libre de Bruxelles, Avenue F.D. Roosevelt 50, CP 169, BE-1050 Brussels, Belgium Email: [email protected] Background and aims – In the context of our preparation of the treatment of the genus Chlorophytum for the ‘Flore d’Afrique centrale’, a new species is described from Burundi and Tanzania. Methods – Herbarium taxonomy and SEM of seeds. Key results – Chlorophytum burundiense Meerts sp. nov. is described. It is a small plant < 35 cm in height, with linear leaves < 6 mm wide, a dense raceme and large, deep purplish brown bracts. It is morphologically not closely related to any other species in the genus. It has a distinct habitat, growing in afromontane grassland and scrub at 2000–2500 m a.s.l. All collections but one originate from Burundi, and a single collection originates from SW Tanzania. A determination key is provided for Chlorophytum species with linear leaves occurring in Burundi. Key words – afromontane, determination key, new species, Chlorophytum, SEM, Burundi, Tanzania. INTRODUCTION silvaticum, C. sparsiflorum, C. stolzii, C. subpetiolatum, C. zingiberastrum (taxonomy and nomenclature after Nordal The circumscription of the genus Chlorophytum Ker Gawl. et al. 1997, Kativu et al. 2008). In addition, a species came (Asparagaceae in APG 2009) was revised by Obermeyer to our attention which could not be identified using Baker (1962), Marais & Reilly (1978), Nordal et al. (1990) and (1898), von Poellnitz (1942, 1946), Nordal et al. -

The Chloroplast Genome of Arthropodium Bifurcatum
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. The Chloroplast Genome of Arthropodium bifurcatum. A thesis presented in partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences Massey University Palmerston North, New Zealand Simon James Lethbridge Cox 2010 Abstract This thesis describes the application of high throughput (Illumina) short read sequencing and analyses to obtain the chloroplast genome sequence of Arthropodium bifurcatum and chloroplast genome markers for future testing of hypotheses that explain geographic distributions of Rengarenga – the name Maori give to species of Arthropodium in New Zealand. It has been proposed that A.cirratum was translocated from regions in the north of New Zealand to zones further south due to its value as a food crop. In order to develop markers to test this hypothesis, the chloroplast genome of the closely related A.bifurcatum was sequenced and annotated. A range of tools were used to handle the large quantities of data produced by the Illumina GAIIx. Programs included the de novo assembler Velvet, alignment tools BWA and Bowtie, the viewer Tablet and the quality control program SolexaQA. The A.bifurcatum genome was then used as a reference to align long range PCR products amplified from multiple accessions of A.cirratum and A.bifurcatum sampled from a range of geographic locations. From this alignment variable SNP markers were identified. -

Insights from Microsporogenesis in Asparagales
EVOLUTION & DEVELOPMENT 9:5, 460–471 (2007) Constraints and selection: insights from microsporogenesis in Asparagales Laurent Penet,a,1,Ã Michel Laurin,b Pierre-Henri Gouyon,a,c and Sophie Nadota aLaboratoire Ecologie, Syste´matique et Evolution, Batiment 360, Universite´ Paris-Sud, 91405 Orsay Ce´dex, France bUMR CNRS 7179, Universite´ Paris 6FPierre & Marie Curie, 2 place Jussieu, Case 7077, 75005 Paris, France cMuse´um National d’Histoire Naturelle, De´partement de Syste´matique et Evolution Botanique, 12 rue Buffon, 75005 Paris CP 39, France ÃAuthor for correspondence (email: [email protected]) 1Current address: Department of Biological Sciences, University of Pittsburgh, 4249 Fifth & Ruskin, Pittsburgh, PA 15260, USA. SUMMARY Developmental constraints have been proposed different characteristics of microsporogenesis, only cell to interfere with natural selection in limiting the available wall formation appeared as constrained. We show that set of potential adaptations. Whereas this concept has constraints may also result from biases in the correlated long been debated on theoretical grounds, it has been occurrence of developmental steps (e.g., lack of successive investigated empirically only in a few studies. In this article, cytokinesis when wall formation is centripetal). We document we evaluate the importance of developmental constraints such biases and their potential outcomes, notably the during microsporogenesis (male meiosis in plants), with an establishment of intermediate stages, which allow emphasis on phylogenetic patterns in Asparagales. Different development to bypass such constraints. These insights are developmental constraints were tested by character discussed with regard to potential selection on pollen reshuffling or by simulated distributions. Among the morphology. INTRODUCTION 1991) also hindered tests using the concept (Pigliucci and Kaplan 2000). -

Phytochemical Screening and Antimicrobial Activity of Chlorophytum Species Leaves of Melghat Region
Available online on www.ijppr.com International Journal of Pharmacognosy and Phytochemical Research 2014; 6(1); 141-145 ISSN: 0975-4873 Research Article Phytochemical Screening and Antimicrobial Activity of Chlorophytum Species Leaves of Melghat Region *Ghorpade D.S1, Thakare P.V.2 1Government College of Pharmacy, Karad 2Department of Biotechnology S G B Amaravati University, Amravati Available online: 1st March 2014 ABSTRACT Aqueous extract of Chlorophytum species leaves of eight plants were screened for in vitro antimicrobial activity using the agar diffusion method. The antimicrobial activity of aqueous extract of leaves of the Chlorophytum species plant was studied against bacteria E. coli, S aureus, P. vulgaris, B.substilis and fungi A.niger, C. albican .Leaves of C. tuberosum show excellent antimicrobial activity against bacteria and fungi tested. Aqueous extract of C. borivilianum, C. arundinaceum, C. nimmoni and C. kolhapurens exhibits good activity against all tested microorganisms while other plants C. breviscapum, C. bharuchae, and C. glaucum showed moderate antimicrobial activity. A zone of inhibition of antibacterial activity compared with standard ampicillin and antifungal with griseofulvin. The preliminary phytochemical screening of leaves reveals the presence of starch, proteins, sugars, tannins, flavonoids, alkaloids and mucilage. Microscopy and Microscopy of transverse section of leaves of Chlorophytum species show characters similarities with the same species Key words: Antibacterial, antifungal, Chlorophytum species, agar diffusion method. INTRODUCTION MATERIALS AND METHODS The numbers of microorganism are becoming drug Plant material: The leaves of plants were collected in rainy resistant to various antibiotics. This lead to increased use season from Melghat region of Amravati District. Plants of broad spectrum antibiotics, immunosuppressive agent, were authenticated by the Botanist, Dr P.A. -

Red Data List Special Edition
Newsletter of the Southern African Botanical Diversity Network Volume 6 No. 3 ISSN 1027-4286 November 2001 Invasive Alien Plants Part 2 Southern Mozambique Expedition Living Plant Collections: Lowveld, Mozambique, Namibia REDSABONET NewsDATA Vol. 6 No. 3 November LIST 2001 SPECIAL EDITION153 c o n t e n t s Red Data List Features Special 157 Profile: Ezekeil Kwembeya ON OUR COVER: 158 Profile: Anthony Mapaura Ferraria schaeferi, a vulnerable 162 Red Data Lists in Southern Namibian near-endemic. 159 Tribute to Paseka Mafa (Photo: G. Owen-Smith) Africa: Past, Present, and Future 190 Proceedings of the GTI Cover Stories 169 Plant Red Data Books and Africa Regional Workshop the National Botanical 195 Herbarium Managers’ 162 Red Data List Special Institute Course 192 Invasive Alien Plants in 170 Mozambique RDL 199 11th SSC Workshop Southern Africa 209 Further Notes on South 196 Announcing the Southern 173 Gauteng Red Data Plant Africa’s Brachystegia Mozambique Expedition Policy spiciformis 202 Living Plant Collections: 175 Swaziland Flora Protection 212 African Botanic Gardens Mozambique Bill Congress for 2002 204 Living Plant Collections: 176 Lesotho’s State of 214 Index Herbariorum Update Namibia Environment Report 206 Living Plant Collections: 178 Marine Fishes: Are IUCN Lowveld, South Africa Red List Criteria Adequate? Book Reviews 179 Evaluating Data Deficient Taxa Against IUCN 223 Flowering Plants of the Criterion B Kalahari Dunes 180 Charcoal Production in 224 Water Plants of Namibia Malawi 225 Trees and Shrubs of the 183 Threatened -

Flora of South Australia 5Th Edition | Edited by Jürgen Kellermann
Flora of South Australia 5th Edition | Edited by Jürgen Kellermann KEY TO FAMILIES1 J.P. Jessop2 The sequence of families used in this Flora follows closely the one adopted by the Australian Plant Census (www.anbg.gov. au/chah/apc), which in turn is based on that of the Angiosperm Phylogeny Group (APG III 2009) and Mabberley’s Plant Book (Mabberley 2008). It differs from previous editions of the Flora, which were mainly based on the classification system of Engler & Gilg (1919). A list of all families recognised in this Flora is printed in the inside cover pages with families already published highlighted in bold. The up-take of this new system by the State Herbarium of South Australia is still in progress and the S.A. Census database (www.flora.sa.gov.au/census.shtml) still uses the old classification of families. The Australian Plant Census web-site presents comparison tables of the old and new systems on family and genus level. A good overview of all families can be found in Heywood et al. (2007) and Stevens (2001–), although these authors accept a slightly different family classification. A number of names with which people using this key may be familiar but are not employed in the system used in this work have been included for convenience and are enclosed on quotation marks. 1. Plants reproducing by spores and not producing flowers (“Ferns and lycopods”) 2. Aerial shoots either dichotomously branched, with scale leaves and 3-lobed sporophores or plants with fronds consisting of a simple or divided sterile blade and a simple or branched spikelike sporophore .................................................................................. -

Comparative Population Dynamics of Two Closely Related Species Differing in Ploidy Level
Comparative Population Dynamics of Two Closely Related Species Differing in Ploidy Level Lucie Cˇ erna´ 1, Zuzana Mu¨ nzbergova´ 1,2* 1 Department of Botany, Faculty of Science, Charles University, Prague, Czech Republic, 2 Institute of Botany, Academy of Sciences, Pru˚honice, Czech Republic Abstract Background: Many studies compare the population dynamics of single species within multiple habitat types, while much less is known about the differences in population dynamics in closely related species in the same habitat. Additionally, comparisons of the effect of habitat types and species are largely missing. Methodology and Principal Findings: We estimated the importance of the habitat type and species for population dynamics of plants. Specifically, we compared the dynamics of two closely related species, the allotetraploid species Anthericum liliago and the diploid species Anthericum ramosum, occurring in the same habitat type. We also compared the dynamics of A. ramosum in two contrasting habitats. We examined three populations per species and habitat type. The results showed that single life history traits as well as the mean population dynamics of A. liliago and A. ramosum from the same habitat type were more similar than the population dynamics of A. ramosum from the two contrasting habitats. Conclusions: Our findings suggest that when transferring knowledge regarding population dynamics between populations, we need to take habitat conditions into account, as these conditions appear to be more important than the species involved (ploidy level). However, the two species differ significantly in their overall population growth rates, indicating that the ploidy level has an effect on species performance. In contrast to what has been suggested by previous studies, we observed a higher population growth rate in the diploid species. -

And Trachyandra Dwaricata
4 PHYTOCHEMICAL INVESTIGATIONS OF BULBINEABYSSINICA, BULBINENATALENSIS AND TRACHYANDRA DWARICATA A THESIS SUBMITTED TO THE SCHOOL OF GRADUATE STUDIES ADDIS ABABA UNIVERSITY IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN CHEMISTRY BY GIZACHEW NIGUSSIE SEPTEMBER, 1999 f dddedicated to: mothery 1Ityij. sister and 7Idty brother* i / ACKNOWLEDGMENTS First and foremost I would like to express my appreciation to my research advisors Dr. Wendimagegn Mammo and Prof. Sebsebe Demissew for their constant guidance and supervision from the conception to the completion of this work. My heartfelt gratitude goes to Prof. Ermias Dagne who provided me with valuable literature sources and authentic samples. I would like to thank all staff members of the Department of Chemistry for contributions they made in one way or the other. I take pleasure in expressing appreciation and thanks to Ato Daniel Bisrat, Ato Zerihun Ayalew, Ato Berhanu Mekonnen, Ato Tesfaye Hailu, Ato Legesse Adane, Ato Alemayehu Mekonnen, Tesfaye Welede and Dawit. I would iike to acknowledge Professor Berhanu Abegaz Molla and through him Network for Analytical and Bioassay Services in Africa (NABSA) for the 300 MHz NMR and mass spectral data. The Department of Organic Chemistry of the Chalmers University of Technology, Gothenburg, Sweden is gratefully acknowledged for the 400 MHz and MS data. I am grateful for the financial and material support from Bahir Dar Polytechnic Institute, and Financial support from the Swedish Agency for Research Cooperation with Developing Countries (SAREC) through the Ethiopian Science and Technology Commission (ESTC) is gratefully acknoweldged. I ' ' W •>!. / / ABSTRACT PHYTOCHEMICAL INVESTIGATIONS OF BULBINE ABYSSINICA, BULBINE NATALENSIS AND TRACHYANDRA DIVARICATA Advisor: Dr. -
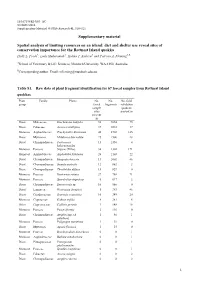
Supplementary Material Spatial Analysis of Limiting Resources on An
10.1071/WR14083_AC ©CSIRO 2014 Supplementary Material: Wildlife Research 41 , 510–521 Supplementary material Spatial analysis of limiting resources on an island: diet and shelter use reveal sites of conservation importance for the Rottnest Island quokka Holly L. Poole A, Laily Mukaromah A, Halina T. Kobryn A and Patricia A. Fleming A,B ASchool of Veterinary & Life Sciences, Murdoch University, WA 6150, Australia. BCorresponding author. Email: [email protected] Table S1. Raw data of plant fragment identification for 67 faecal samples from Rottnest Island quokkas Plant Family Plants No. No. No. field group faecal fragments validation sample quadrats sites present in present in Dicot Malvaceae Guichenotia ledifolia 52 9854 75 Dicot Fabaceae Acacia rostellifera 37 3018 37 Monocot Asphodelaceae Trachyandra divaricata 46 2702 145 Dicot Myrtaceae Melaleuca lanceolata 25 1506 28 Dicot Chenopodiaceae Tecticornia 13 1350 4 halocnemoides Monocot Poaceae Stipeae (Tribe) 34 1302 171 Monocot Asphodelaceae Asphodelus fistulosus 26 1103 22 Dicot Chenopodiaceae Rhagodia baccata 13 1002 46 Dicot Chenopodiaceae Suaeda australis 12 862 2 Dicot Chenopodiaceae Threlkeldia diffusa 15 829 0 Monocot Poaceae Rostraria cristata 27 788 71 Monocot Poaceae Sporobolus virginicus 5 617 2 Dicot Chenopodiaceae Sarcocornia sp . 10 560 0 Dicot Lamiaceae Westringia dampieri 5 383 46 Dicot Goodeniaceae Scaevola crassifolia 10 349 20 Monocot Cyperaceae Gahnia trifida 8 281 6 Other Cupressaceae Callitris preissii 3 148 18 Monocot Poaceae Poa poiformis 2 116 0 Dicot Chenopodiaceae Atriplex spp. (A. 1 40 1 paludosa ) Monocot Poaceae Polypogon maritimus 1 39 0 Dicot Myrtaceae Agonis flexuosa 1 15 0 Monocot Poaceae Brachypodium distachyon 0 0 1 Monocot Asphodelaceae Bulbine semibarbata 0 0 1 Dicot Pittosporaceae Pittosporum 0 0 1 phylliraeoides Monocot Poaceae Spinifex longifolius 0 0 1 Dicot Fabaceae Acacia saligna 0 0 2 Dicot Chenopodiaceae Atriplex cinerea 0 0 2 1 Dicot Asteraceae Centaurea sp . -

Vascular Plants of Negelle-Borona Kallos
US Forest Service Technical Assistance Trip Federal Democratic Republic of Ethiopia In Support to USAID-Ethiopia for Assistance in Rangeland Management Support to the Pastoralist Livelihoods Initiative for USAID-Ethiopia Office of Business Environment Agriculture & Trade Vascular Plants of Negelle-Borona Kallos Mission dates: November 19 to December 21, 2011 Report submitted June 6, 2012 by Karen L. Dillman, Ecologist USDA Forest Service, Tongass National Forest [email protected] Vascular Plants of Negelle-Borona, Ethiopia, USFS IP Introduction This report provides supplemental information to the Inventory and Assessment of Biodiversity report prepared for the US Agency for International Development (USAID) following the 2011 mission to Negelle- Borona region in southern Ethiopia (Dillman 2012). As part of the USAID supported Pastoralist Livelihood Initiative (PLI), this work focused on the biodiversity of the kallos (pastoral reserves). This report documents the vascular plant species collected and identified from in and around two kallos near Negelle (Oda Yabi and Kare Gutu). This information can be utilized to develop a comprehensive plant species list for the kallos which will be helpful in future vegetation monitoring and biodiversity estimates in other locations of the PLI project. This list also identifies plants that are endemic to Ethiopia and East Africa growing in the kallos as well as plants that are non-native and could be considered invasive in the rangelands. Methods Field work was conducted between November 28 and December 9, 2011 (the end of the short rainy season). The rangeland habitats visited are dominated by Acacia and Commifera trees, shrubby Acacia or dwarf shrub grasslands.