Filed Comments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -

(12) United States Patent (10) Patent No.: US 8,603,526 B2 Tygesen Et Al
USOO8603526B2 (12) United States Patent (10) Patent No.: US 8,603,526 B2 Tygesen et al. (45) Date of Patent: Dec. 10, 2013 (54) PHARMACEUTICAL COMPOSITIONS 2008. O152595 A1 6/2008 Emigh et al. RESISTANT TO ABUSE 2008. O166407 A1 7/2008 Shalaby et al. 2008/0299.199 A1 12/2008 Bar-Shalom et al. 2008/0311205 A1 12/2008 Habib et al. (75) Inventors: Peter Holm Tygesen, Smoerum (DK); 2009/0022790 A1 1/2009 Flath et al. Jan Martin Oevergaard, Frederikssund 2010/0203129 A1 8/2010 Andersen et al. (DK); Karsten Lindhardt, Haslev (DK); 2010/0204259 A1 8/2010 Tygesen et al. Louise Inoka Lyhne-versen, Gentofte 2010/0239667 A1 9/2010 Hemmingsen et al. (DK); Martin Rex Olsen, Holbaek 2010, O291205 A1 11/2010 Downie et al. (DK); Anne-Mette Haahr, Birkeroed 2011 O159100 A1 6/2011 Andersen et al. (DK); Jacob Aas Hoellund-Jensen, FOREIGN PATENT DOCUMENTS Frederikssund (DK); Pemille Kristine Hoeyrup Hemmingsen, Bagsvaerd DE 20 2006 014131 1, 2007 (DK) EP O435,726 8, 1991 EP O493513 7, 1992 EP O406315 11, 1992 (73) Assignee: Egalet Ltd., London (GB) EP 1213014 6, 2002 WO WO 89,09066 10, 1989 (*) Notice: Subject to any disclaimer, the term of this WO WO91,040 15 4f1991 patent is extended or adjusted under 35 WO WO95/22962 8, 1995 U.S.C. 154(b) by 489 days. WO WO99,51208 10, 1999 WO WOOOf 41704 T 2000 WO WO 03/024426 3, 2003 (21) Appl. No.: 12/701,429 WO WOO3,O24429 3, 2003 WO WOO3,O24430 3, 2003 (22) Filed: Feb. -

1,1,1-Trichloroethane (CASRN 71-55-6) | IRIS
Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment 1,1,1-Trichloroethane; CASRN 71-55-6 Human health assessment information on a chemical substance is included in the IRIS database only after a comprehensive review of toxicity data, as outlined in the IRIS assessment development process. Sections I (Health Hazard Assessments for Noncarcinogenic Effects) and II (Carcinogenicity Assessment for Lifetime Exposure) present the conclusions that were reached during the assessment development process. Supporting information and explanations of the methods used to derive the values given in IRIS are provided in the guidance documents located on the IRIS website. STATUS OF DATA FOR 1,1,1-Trichloroethane File First On-Line 03/31/1987 Category (section) Assessment Available? Last Revised Oral RfD (I.A.) Acute Oral RfD (I.A.1.) qualitative discussion 09/28/2007 Short-term Oral RfD (I.A.2.) qualitative discussion 09/28/2007 Subchronic Oral RfD (I.A.3.) yes 09/28/2007 Chronic Oral RfD (I.A.4.) yes 09/28/2007 Inhalation RfC (I.B.) Acute Inhalation RfC (I.B.1.) yes 09/28/2007 Short-term Inhalation RfC (I.B.2.) yes 09/28/2007 Subchronic Inhalation RfC (I.B.3.) yes 09/28/2007 1 Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment Category (section) Assessment Available? Last Revised Chronic Inhalation RfC (I.B.4.) yes 09/28/2007 Carcinogenicity Assessment (II.) yes 09/28/2007 I. Health Hazard Assessments for Noncarcinogenic Effects I.A. -

Pharmacological Classification of the Abuse-Related Discriminative
Ashdin Publishing Journal of Drug and Alcohol Research ASHDIN Vol. 3 (2014), Article ID 235839, 10 pages publishing doi:10.4303/jdar/235839 Research Article Pharmacological Classification of the Abuse-Related Discriminative Stimulus Effects of Trichloroethylene Vapor Keith L. Shelton and Katherine L. Nicholson Department of Pharmacology and Toxicology, School of Medicine, Virginia Commonwealth University, P.O. Box 980613, Richmond, VA 23298, USA Address correspondence to Keith L. Shelton, [email protected] Received 10 December 2013; Revised 2 January 2014; Accepted 27 January 2014 Copyright © 2014 Keith L. Shelton and Katherine L. Nicholson. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Inhalants are distinguished as a class primarily based upon common means of administration suggests a homogeneity of a shared route of administration. Grouping inhalants according to mechanism and behavioral effects that may be scientifically their abuse-related in vivo pharmacological effects using the drug unwarranted. As an illustration of the inadequacy of this discrimination procedure has the potential to provide a more relevant classification scheme to the research and treatment community. simplistic taxonomic approach, consider the fact that if a Mice were trained to differentiate the introceptive effects of the similar system was applied to other common drugs of abuse, trichloroethylene vapor from air using an operant procedure. then tobacco, crack cocaine, and marijuana would be treated Trichloroethylene is a chlorinated hydrocarbon solvent once used as a single category. Instead, other classes of abused drugs as an anesthetic as well as in glues and other consumer products. -

Physiologically-Based Pharmacokinetic Modelling of Infant
AAS Open Research AAS Open Research 2018, 1:16 Last updated: 21 MAY 2018 RESEARCH ARTICLE Physiologically-based pharmacokinetic modelling of infant exposure to efavirenz through breastfeeding [version 1; referees: 1 approved with reservations] Adeniyi Olagunju 1,2, Rajith K. R. Rajoli 2, Shakir A. Atoyebi1, Saye Khoo2, Andrew Owen2, Marco Siccardi2 1Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria 2Department of Molecular and Clinical Pharmacology, University of Liverpool, Liverpool, L69 3GF, UK v1 First published: 14 May 2018, 1:16 (doi: 10.12688/aasopenres.12860.1) Open Peer Review Latest published: 14 May 2018, 1:16 (doi: 10.12688/aasopenres.12860.1) Referee Status: Abstract Background: Very little is known about the level of infant exposure to many drugs commonly used during breastfeeding. The aim of this study was to Invited Referees develop a physiologically-based pharmacokinetic (PBPK) model for predicting 1 infant exposure to maternal efavirenz through breastmilk. Methods: A breastfeeding PBPK model combining whole-body maternal and version 1 infant sub-models was constructed from drug-specific and system parameters published report affecting drug disposition using mathematical descriptions. The model was 14 May 2018 validated against published data on the pharmacokinetics of efavirenz in nursing mother-infant pairs. Further simulations were conducted to assess 1 Jeffrey W. Fisher, US Food and Drug exposure in the context of the 400 mg reduced dose of efavirenz as well as Administration, USA best- and worse-case scenarios. Results: The model adequately described efavirenz pharmacokinetics, with over 80% of observed data points (203 matched breast milk and plasma pairs) Discuss this article within the predictive interval. -

Federal Register/Vol. 71, No. 34/Tuesday, February 21, 2006
Federal Register / Vol. 71, No. 34 / Tuesday, February 21, 2006 / Notices 8859 DEPARTMENT OF HEALTH AND DEPARTMENT OF HEALTH AND of the Public Health Service Act to HUMAN SERVICES HUMAN SERVICES conduct directly or by grants or contracts, research, experiments, and Office of the National Coordinator; Office of the National Coordinator; demonstrations relating to occupational American Health Information American Health Information safety and health and to mine health. Community Chronic Care Workgroup Community Consumer Empowerment The BSC shall provide guidance to the Meeting Workgroup Meeting Director, NIOSH on research and prevention programs. Specifically, the ACTION: Announcement of meeting. ACTION: Announcement of meeting. board shall provide guidance on the institute’s research activities related to SUMMARY: SUMMARY: This notice announces the This notice announces the developing and evaluating hypotheses, third meeting of the American Health third meeting of the American Health Information Community Consumer systematically documenting findings Information Community Chronic Care and disseminating results. The board Workgroup in accordance with the Empowerment Workgroup in accordance with the Federal Advisory shall evaluate the degree to which the Federal Advisory Committee Act (Pub. activities of NIOSH: (1) Conform to L. 92–463, 5 U.S.C., App.) Committee Act (Pub. L. 92–463, 5 U.S.C., App.) appropriate scientific standards, (2) DATES: March 22, 2006 from 1 p.m. to address current, relevant needs, and (3) DATES: March 20, 2006 from 1 p.m. to 5 p.m. produce intended results. 5 p.m. ADDRESSES: Hubert H. Humphrey Matters to be Discussed: Agenda items ADDRESSES: Hubert H. Humphrey Building (200 Independence Ave., SW., include a report from the Director, Building (200 Independence Ave., SW., Washington, DC 20201), Conference NIOSH; progress report by BSC working Washington, DC 20201), Conference Room 705A. -
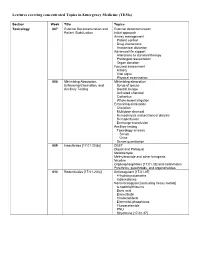
Lectures Covering Concentrated Topics in Emergency Medicine (Tems)
Lectures covering concentrated Topics in Emergency Medicine (TEMs) Section Week Title Topics Toxicology 007 External Decontamination and External decontamination Patient Stabilization Initial approach Airway management Patient control Drug interactions Anatomical distortion Advanced life support Alterations to standard therapy Prolonged resuscitation Organ donation Focused assessment History Vital signs Physical examination 008 Minimizing Absorption, Minimizing absorption Enhancing Elimination, and Syrup of ipecac Ancillary Testing Gastric lavage Activated charcoal Cathartics Whole-bowel irrigation Enhancing elimination Chelation Multidose charcoal Hemodialysis and peritoneal dialysis Hemoperfusion Exchange transfusion Ancillary testing Toxicology screens Serum Urine Serum quantitation 009 Insecticides [17.01.20(b)] DEET Diquat and Paraquat Metaldehyde Methylbromide and other fumigants Nicotine Organophosphates [17.01.33] and carbamates Pyrethrins, pyrethroids, and organohalides 010 Rodenticides [17.01.20(c)] Anticoagulant [17.01.05] 4-hydroxycoumarins Indanediones Nonanticoagulant [excluding heavy metals] α-naphthylthiourea Boric acid Bromethalin Cholecalciferol Elemental phosphorus Fluroacetamide PNU Strychnine [17.01.37] 011 Hydrocarbons [17.01.23] Aliphatic Aromatic General Toluene Xylene Halogenated General Carbon tetrachloride Methylene chloride Trichloroethylene Pine Oil and Turpentine Polychlorinated biphenyls and related substances 012 Caustic & Corrosive Agents Ingestions Acid [17.01.14(a)] Alkali [17.01.14(b)] Other Disc batteries -

BYSTOLIC Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION • Patients with severe hepatic impairment (Child-Pugh >B) These highlights do not include all the information needed to (4) use BYSTOLIC safely and effectively. See full prescribing • Hypersensitive to any component of this product (4) information for BYSTOLIC. -------------WARNINGS AND PRECAUTIONS---------- BYSTOLIC® (nebivolol) tablets, for oral use • Acute exacerbation of coronary artery disease upon cessation of therapy: Do not abruptly discontinue. (5.1) Initial U.S. Approval: 2007 • Diabetes: Monitor glucose as β-blockers may mask symptoms of hypoglycemia. (5.5) ------------RECENT MAJOR CHANGES------------------ Indications and Usage (1.1) 12/2011 -----------------ADVERSE REACTIONS----------------- Most common adverse reactions (6.1): --------------INDICATIONS AND USAGE--------------- • Headache, fatigue BYSTOLIC is a beta-adrenergic blocking agent indicated for To report SUSPECTED ADVERSE REACTIONS, Contact the treatment of hypertension, to lower blood pressure. Forest Laboratories, Inc. at 1-800-678-1605 or FDA at Lowering blood pressure reduces the risk of fatal and nonfatal 1-800-FDA-1088 or www.fda.gov/medwatch. cardiovascular events, primarily strokes and myocardial infarctions. (1.1) ----------------DRUG INTERACTIONS------------------ • CYP2D6 enzyme inhibitors may increase nebivolol levels. ------------DOSAGE AND ADMINISTRATION---------- (7.1) Can be taken with and without food. Individualize to the • Reserpine or clonidine may produce excessive reduction needs of the patient and monitor during up-titration. (2) of sympathetic activity. (7.2) • Hypertension: Most patients start at 5 mg once daily. • Both digitalis glycosides and β-blockers slow Dose can be increased at 2-week intervals up to 40 mg. atrioventricular conduction and decrease heart rate. (2.1) Concomitant use can increase the risk of bradycardia. -

Friendly Reference Document for Hazardous Waste Characteristics
Hazardous Waste Characteristics A User-Friendly Reference Document October 2009 1 Table of Contents Introduction..................................................................................................................................... 3 Overview of the Hazardous Waste Identification Process .......................................................... 5 Characteristic Hazardous Wastes ................................................................................................ 5 Summary Chart............................................................................................................................ 7 General Hazardous Waste Resources.......................................................................................... 8 Regulations for the Identification of Hazardous Waste............................................................... 9 General Resources for Hazardous Waste Characteristics.......................................................... 12 Ignitability..................................................................................................................................... 12 Regulations for Ignitable Liquids.............................................................................................. 13 Regulations for Ignitable Solids ................................................................................................ 14 Regulations for Ignitable Compressed Gas ............................................................................... 14 Related Resources for Ignitable -

Basic Analytical Toxicology
The World Health Organization is a specialized agency of the United Nations with primary responsibility for international health matters and public health. Through this organization, which was created in 1948, the health professions of some 190 countries exchange their knowledge and experience with the aim of making possible the attainment by all citizens of the world by the year 2000 of a level of health that will permit them to lead a socially and economically productive life. By means of direct technical cooperation with its Member States, and by stimulating such coopera tion among them, WHO promotes the development of comprehensive health services, the prevention and control of diseases, the improvement of environmental conditions, the development of human resources for health, the coordination and development of biomedical and health services research, and the planning and implementation of health programmes. These broad fields of endeavour encompass a wide variety of activities, such as developing systems of primary health care that reach the whole population of Member countries; promoting the health of mothers and children; combating malnutrition, controlling malaria and other communicable diseases including tuberculosis and leprosy; coordinating the global strategy for the prevention and control of AIDS; having achieved the eradication of smallpox, promoting mass immunization against a number of other preventable diseases; improving mental health; providing safe water supplies; and training health personnel of all categories. Progress towards better health throughout the world also demands international cooperation in such matters as establishing international standards for biological substances, pesticides and pharma ceuticals; formulating environmental health criteria; recommending international nonproprietary names for drugs; administering the International Health Regulations; revising the International Statistical Classification of Diseases and Related Health Problems and collecting and disseminating healih statistical information. -

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances
.. ~~.~~.~ INTERNATIONAL NARCOTICS CONTROL BOARD Vienna ~\\ ~~C:~Ob PRECURSORS AND CHEMICALS FREQUENTLY USED IN THE ILLICIT MANUFACTURE OF NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES Report of the International Narcotics Control Board for 1995 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 ~, \rg;}j ~~ UNITED NATIONS REPORTS PUBLISHED BY THE INTERNATIONAL NARCOTICS CONTROL BOARD IN 1995 The Report ofthe International Narcotics Control Boardfor 1995 (FJINCB/1995/1) is supplemented by the following technical reports: Narcotic Drugs: Estimated World Requirements for 1996; Statistics for 1994 (FJINCB/ 1995/2) Psychotropic Substances: Statistics for 1994; Assessments of Medical and Scientific Requirements for Substances in Schedules /I, /11 and IV; Requirement of Import Authorizations for Substances in Schedules /11 and IV (E1INCB/1995/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 1995 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (FJINCB/1995/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms ("Yellow List", "Green List" and "Red List"), which are also issued by the Board. CONTACTING THE INTERNATIONAL NARCOTICS CONTROL BOARD The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1313 P.O. -

Solvents/Inhalants Information for Health Professionals
Solvents/Inhalants Information for Health Professionals Introduction Inhalants compromise a wide variety of vapours, gases, and aerosols that can be inhaled to induce psychoactive (mind-altering) effects. Unlike other drugs, inhalants are available as legal products with low costs and wide availability. Examples include commercial products such as nail polish remover, hair sprays, lighter fluid, cleaning fluids, vegetable frying pan lubricants, and spray paints. These chemicals can be sniffed or inhaled because they are gaseous at room temperature and pressure. “Inhalant” is a general term that includes all substances used in this way. Most inhalants are volatile solvents, which are liquids that easily vaporize at room temperature and can contain many different chemicals that may be psychoactive. The majority of these solvents are produced from petroleum and natural gas. They have an enormous number of industrial, commercial and household uses, and are found in automobile fuels, cleaning fluids, toiletries, adhesives and fillers, paints, paint thinners, felt-tip markers, and many other products. In addition to volatile solvents, aerosols (hair spray, paint spray) can be abused as inhalants. Other inhalants include the nitrites (amyl and butyl, “poppers”, “Rush”) and gases such as the anesthetics nitrous oxide (laughing gas) and ether. Inhalants can be breathed in through the nose or mouth in a variety of ways, including spraying aerosols directly into the nose or mouth, “sniffing” or "snorting” fumes from containers, inhaling from balloons filled with nitrous oxide, “huffing” from an inhalant-soaked rag stuffed in the mouth, or “bagging”- pouring the substance over a cloth or into a plastic bag and breathing in the vapours.