Occupational Exposure to Isoflurane Anesthetic Gas in the Research Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Veterinary Emergency & Anaesthesia Pfizer
AVA ECVA & AVEF Thank all their sponsors for this spring edition PARIS 2007 On VETERINARY EMERGENCY & ANAESTHESIA PFIZER MERIAL FORT DODGE BAYER BOEHRINGER MEDISUR COVETO OPTOMED HALLOWELL SCIL JANSSEN SOGEVAL KRUSSE TECHNIBELT MILA TEM The Organisatiors 7th AVEF European Meeting- 10th March 2007-ROISSY 2 AVA – ECVA Spring Meeting 2007 on Veterinary Emergency & Anesthesia 7 – 10 March 2007, Paris, France AVA PARIS 2007 — Wednesday March 7th RESIDENT DAY RUMINANT ANAESTHESIA Hyatt Regency Hotel, Roissy CDG, France K OTTO, D HOLOPHERNE, G TOUZOT 8.30 REGISTRATIONS 9.00-9.45 Specific anatomo-physiology to consider for ruminant peri-anaesthetic period K OTTO 10.00-10.30 COFFEE BREAK 10.30-11.15 Post-anaesthetic and pain management in ruminants K OTTO 11.30-12.15 Physical restraint and sedation of ruminants D HOLOPHERNE 12.30-1.30 LUNCH 1.30-2.15 Anaesthesia of Lamas & Alpagas G TOUZOT 2.30-3.15 Regional & local anaesthesia for ruminants D HOLOPHERNE 3.30-4.00 COFFEE BREAK 4.00-4.45 Pharmacology and protocols for ruminant anaesthesia G TOUZOT AVA-ECVA PARIS 2007, Veterinary Emergency & Anaesthesia, 7-10th March AVA-ECVA PARIS 2007, Veterinary Emergency & Anaesthesia, 7-10th March AVA – ECVA Spring Meeting 2007 on Veterinary Emergency & Anesthesia 7 – 10 March 2007, Paris, France Specific anatomo-physiology to consider for ruminants peri-anaesthetic period Klaus A. Otto Institut für Versuchstierkunde und Zentrales Tierlaboratorium, Medizinische Hochschule Hannover, D-30623 Hannover, Germany The suborder “ruminantia” includes members of the family “bovidae” such as cattle (bos taurus), sheep (ovis spp) and goats (capra spp). Members of the family “camelidae” (camelus spp, llama spp, vicugna spp) belong to the suborder “tylopodia” and therefore are not true ruminants. -

Evaluating the Translational Potential of Progesterone Treatment Following
Wong et al. BMC Neuroscience 2014, 15:131 http://www.biomedcentral.com/1471-2202/15/131 RESEARCH ARTICLE Open Access Evaluating the translational potential of progesterone treatment following transient cerebral ischaemia in male mice Raymond Wong1, Claire L Gibson2*, David A Kendall3 and Philip MW Bath1 Abstract Background: Progesterone is neuroprotective in numerous preclinical CNS injury models including cerebral ischaemia. The aim of this study was two-fold; firstly, we aimed to determine whether progesterone delivery via osmotic mini-pump would confer neuroprotective effects and whether such neuroprotection could be produced in co-morbid animals. Results: Animals underwent transient middle cerebral artery occlusion. At the onset of reperfusion, mice were injected intraperitoneally with progesterone (8 mg/kg in dimethylsulfoxide). Adult and aged C57 Bl/6 mice were dosed additionally with subcutaneous infusion (1.0 μl/h of a 50 mg/ml progesterone solution) via implanted osmotic minipumps. Mice were allowed to survive for up to 7 days post-ischaemia and assessed for general well-being (mass loss and survival), neurological score, foot fault and t-maze performance. Progesterone reduced neurological deficit [F(1,2) = 5.38, P = 0.027] and number of contralateral foot-faults [F(1,2) = 7.36, P = 0.0108] in adult, but not aged animals, following ischaemia. In hypertensive animals, progesterone treatment lowered neurological deficit [F(1,6) = 18.31, P = 0.0001], reduced contralateral/ipsilateral alternation ratio % [F(1,2) = 17.05, P = 0.0006] and time taken to complete trials [F(1,2) = 15.92, P = 0.0009] for t-maze. Conclusion: Post-ischemic progesterone administration via mini-pump delivery is effective in conferring functional improvement in a transient MCAO model in adult mice. -

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -

(12) United States Patent (10) Patent No.: US 8,603,526 B2 Tygesen Et Al
USOO8603526B2 (12) United States Patent (10) Patent No.: US 8,603,526 B2 Tygesen et al. (45) Date of Patent: Dec. 10, 2013 (54) PHARMACEUTICAL COMPOSITIONS 2008. O152595 A1 6/2008 Emigh et al. RESISTANT TO ABUSE 2008. O166407 A1 7/2008 Shalaby et al. 2008/0299.199 A1 12/2008 Bar-Shalom et al. 2008/0311205 A1 12/2008 Habib et al. (75) Inventors: Peter Holm Tygesen, Smoerum (DK); 2009/0022790 A1 1/2009 Flath et al. Jan Martin Oevergaard, Frederikssund 2010/0203129 A1 8/2010 Andersen et al. (DK); Karsten Lindhardt, Haslev (DK); 2010/0204259 A1 8/2010 Tygesen et al. Louise Inoka Lyhne-versen, Gentofte 2010/0239667 A1 9/2010 Hemmingsen et al. (DK); Martin Rex Olsen, Holbaek 2010, O291205 A1 11/2010 Downie et al. (DK); Anne-Mette Haahr, Birkeroed 2011 O159100 A1 6/2011 Andersen et al. (DK); Jacob Aas Hoellund-Jensen, FOREIGN PATENT DOCUMENTS Frederikssund (DK); Pemille Kristine Hoeyrup Hemmingsen, Bagsvaerd DE 20 2006 014131 1, 2007 (DK) EP O435,726 8, 1991 EP O493513 7, 1992 EP O406315 11, 1992 (73) Assignee: Egalet Ltd., London (GB) EP 1213014 6, 2002 WO WO 89,09066 10, 1989 (*) Notice: Subject to any disclaimer, the term of this WO WO91,040 15 4f1991 patent is extended or adjusted under 35 WO WO95/22962 8, 1995 U.S.C. 154(b) by 489 days. WO WO99,51208 10, 1999 WO WOOOf 41704 T 2000 WO WO 03/024426 3, 2003 (21) Appl. No.: 12/701,429 WO WOO3,O24429 3, 2003 WO WOO3,O24430 3, 2003 (22) Filed: Feb. -

1,1,1-Trichloroethane (CASRN 71-55-6) | IRIS
Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment 1,1,1-Trichloroethane; CASRN 71-55-6 Human health assessment information on a chemical substance is included in the IRIS database only after a comprehensive review of toxicity data, as outlined in the IRIS assessment development process. Sections I (Health Hazard Assessments for Noncarcinogenic Effects) and II (Carcinogenicity Assessment for Lifetime Exposure) present the conclusions that were reached during the assessment development process. Supporting information and explanations of the methods used to derive the values given in IRIS are provided in the guidance documents located on the IRIS website. STATUS OF DATA FOR 1,1,1-Trichloroethane File First On-Line 03/31/1987 Category (section) Assessment Available? Last Revised Oral RfD (I.A.) Acute Oral RfD (I.A.1.) qualitative discussion 09/28/2007 Short-term Oral RfD (I.A.2.) qualitative discussion 09/28/2007 Subchronic Oral RfD (I.A.3.) yes 09/28/2007 Chronic Oral RfD (I.A.4.) yes 09/28/2007 Inhalation RfC (I.B.) Acute Inhalation RfC (I.B.1.) yes 09/28/2007 Short-term Inhalation RfC (I.B.2.) yes 09/28/2007 Subchronic Inhalation RfC (I.B.3.) yes 09/28/2007 1 Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment Category (section) Assessment Available? Last Revised Chronic Inhalation RfC (I.B.4.) yes 09/28/2007 Carcinogenicity Assessment (II.) yes 09/28/2007 I. Health Hazard Assessments for Noncarcinogenic Effects I.A. -

FORANE (Isoflurane, USP)
Forane ® (isoflurane, USP) Proposed Package Insert FORANE (isoflurane, USP) Liquid For Inhalation Rx only DESCRIPTION FORANE (isoflurane, USP), a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 1-chloro-2, 2,2-trifluoroethyl difluoromethyl ether, and its structural formula is: Some physical constants are: Molecular weight 184.5 Boiling point at 760 mm Hg 48.5°C (uncorr.) 20 1.2990-1.3005 Refractive index n D Specific gravity 25°/25°C 1.496 Vapor pressure in mm Hg** 20°C 238 25°C 295 30°C 367 35°C 450 **Equation for vapor pressure calculation: log10Pvap = A + B where A = 8.056 T B = -1664.58 T = °C + 273.16 (Kelvin) Partition coefficients at 37°C: Water/gas 0.61 Blood/gas 1.43 Oil/gas 90.8 1 Forane ® (isoflurane, USP) Proposed Package Insert Partition coefficients at 25°C – rubber and plastic Conductive rubber/gas 62.0 Butyl rubber/gas 75.0 Polyvinyl chloride/gas 110.0 Polyethylene/gas ~2.0 Polyurethane/gas ~1.4 Polyolefin/gas ~1.1 Butyl acetate/gas ~2.5 Purity by gas >99.9% chromatography Lower limit of None flammability in oxygen or nitrous oxide at 9 joules/sec. and 23°C Lower limit of Greater than useful concentration in flammability in oxygen anesthesia. or nitrous oxide at 900 joules/sec. and 23°C Isoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers. Isoflurane has a mildly pungent, musty, ethereal odor. Samples stored in indirect sunlight in clear, colorless glass for five years, as well as samples directly exposed for 30 hours to a 2 amp, 115 volt, 60 cycle long wave U.V. -

Pharmacological Classification of the Abuse-Related Discriminative
Ashdin Publishing Journal of Drug and Alcohol Research ASHDIN Vol. 3 (2014), Article ID 235839, 10 pages publishing doi:10.4303/jdar/235839 Research Article Pharmacological Classification of the Abuse-Related Discriminative Stimulus Effects of Trichloroethylene Vapor Keith L. Shelton and Katherine L. Nicholson Department of Pharmacology and Toxicology, School of Medicine, Virginia Commonwealth University, P.O. Box 980613, Richmond, VA 23298, USA Address correspondence to Keith L. Shelton, [email protected] Received 10 December 2013; Revised 2 January 2014; Accepted 27 January 2014 Copyright © 2014 Keith L. Shelton and Katherine L. Nicholson. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Inhalants are distinguished as a class primarily based upon common means of administration suggests a homogeneity of a shared route of administration. Grouping inhalants according to mechanism and behavioral effects that may be scientifically their abuse-related in vivo pharmacological effects using the drug unwarranted. As an illustration of the inadequacy of this discrimination procedure has the potential to provide a more relevant classification scheme to the research and treatment community. simplistic taxonomic approach, consider the fact that if a Mice were trained to differentiate the introceptive effects of the similar system was applied to other common drugs of abuse, trichloroethylene vapor from air using an operant procedure. then tobacco, crack cocaine, and marijuana would be treated Trichloroethylene is a chlorinated hydrocarbon solvent once used as a single category. Instead, other classes of abused drugs as an anesthetic as well as in glues and other consumer products. -

Veterinary Anesthetic and Analgesic Formulary 3Rd Edition, Version G
Veterinary Anesthetic and Analgesic Formulary 3rd Edition, Version G I. Introduction and Use of the UC‐Denver Veterinary Formulary II. Anesthetic and Analgesic Considerations III. Species Specific Veterinary Formulary 1. Mouse 2. Rat 3. Neonatal Rodent 4. Guinea Pig 5. Chinchilla 6. Gerbil 7. Rabbit 8. Dog 9. Pig 10. Sheep 11. Non‐Pharmaceutical Grade Anesthetics IV. References I. Introduction and Use of the UC‐Denver Formulary Basic Definitions: Anesthesia: central nervous system depression that provides amnesia, unconsciousness and immobility in response to a painful stimulation. Drugs that produce anesthesia may or may not provide analgesia (1, 2). Analgesia: The absence of pain in response to stimulation that would normally be painful. An analgesic drug can provide analgesia by acting at the level of the central nervous system or at the site of inflammation to diminish or block pain signals (1, 2). Sedation: A state of mental calmness, decreased response to environmental stimuli, and muscle relaxation. This state is characterized by suppression of spontaneous movement with maintenance of spinal reflexes (1). Animal anesthesia and analgesia are crucial components of an animal use protocol. This document is provided to aid in the design of an anesthetic and analgesic plan to prevent animal pain whenever possible. However, this document should not be perceived to replace consultation with the university’s veterinary staff. As required by law, the veterinary staff should be consulted to assist in the planning of procedures where anesthetics and analgesics will be used to avoid or minimize discomfort, distress and pain in animals (3, 4). Prior to administration, all use of anesthetics and analgesic are to be approved by the Institutional Animal Care and Use Committee (IACUC). -

Physiologically-Based Pharmacokinetic Modelling of Infant
AAS Open Research AAS Open Research 2018, 1:16 Last updated: 21 MAY 2018 RESEARCH ARTICLE Physiologically-based pharmacokinetic modelling of infant exposure to efavirenz through breastfeeding [version 1; referees: 1 approved with reservations] Adeniyi Olagunju 1,2, Rajith K. R. Rajoli 2, Shakir A. Atoyebi1, Saye Khoo2, Andrew Owen2, Marco Siccardi2 1Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria 2Department of Molecular and Clinical Pharmacology, University of Liverpool, Liverpool, L69 3GF, UK v1 First published: 14 May 2018, 1:16 (doi: 10.12688/aasopenres.12860.1) Open Peer Review Latest published: 14 May 2018, 1:16 (doi: 10.12688/aasopenres.12860.1) Referee Status: Abstract Background: Very little is known about the level of infant exposure to many drugs commonly used during breastfeeding. The aim of this study was to Invited Referees develop a physiologically-based pharmacokinetic (PBPK) model for predicting 1 infant exposure to maternal efavirenz through breastmilk. Methods: A breastfeeding PBPK model combining whole-body maternal and version 1 infant sub-models was constructed from drug-specific and system parameters published report affecting drug disposition using mathematical descriptions. The model was 14 May 2018 validated against published data on the pharmacokinetics of efavirenz in nursing mother-infant pairs. Further simulations were conducted to assess 1 Jeffrey W. Fisher, US Food and Drug exposure in the context of the 400 mg reduced dose of efavirenz as well as Administration, USA best- and worse-case scenarios. Results: The model adequately described efavirenz pharmacokinetics, with over 80% of observed data points (203 matched breast milk and plasma pairs) Discuss this article within the predictive interval. -

Federal Register/Vol. 71, No. 34/Tuesday, February 21, 2006
Federal Register / Vol. 71, No. 34 / Tuesday, February 21, 2006 / Notices 8859 DEPARTMENT OF HEALTH AND DEPARTMENT OF HEALTH AND of the Public Health Service Act to HUMAN SERVICES HUMAN SERVICES conduct directly or by grants or contracts, research, experiments, and Office of the National Coordinator; Office of the National Coordinator; demonstrations relating to occupational American Health Information American Health Information safety and health and to mine health. Community Chronic Care Workgroup Community Consumer Empowerment The BSC shall provide guidance to the Meeting Workgroup Meeting Director, NIOSH on research and prevention programs. Specifically, the ACTION: Announcement of meeting. ACTION: Announcement of meeting. board shall provide guidance on the institute’s research activities related to SUMMARY: SUMMARY: This notice announces the This notice announces the developing and evaluating hypotheses, third meeting of the American Health third meeting of the American Health Information Community Consumer systematically documenting findings Information Community Chronic Care and disseminating results. The board Workgroup in accordance with the Empowerment Workgroup in accordance with the Federal Advisory shall evaluate the degree to which the Federal Advisory Committee Act (Pub. activities of NIOSH: (1) Conform to L. 92–463, 5 U.S.C., App.) Committee Act (Pub. L. 92–463, 5 U.S.C., App.) appropriate scientific standards, (2) DATES: March 22, 2006 from 1 p.m. to address current, relevant needs, and (3) DATES: March 20, 2006 from 1 p.m. to 5 p.m. produce intended results. 5 p.m. ADDRESSES: Hubert H. Humphrey Matters to be Discussed: Agenda items ADDRESSES: Hubert H. Humphrey Building (200 Independence Ave., SW., include a report from the Director, Building (200 Independence Ave., SW., Washington, DC 20201), Conference NIOSH; progress report by BSC working Washington, DC 20201), Conference Room 705A. -

Newborn Anaesthesia: Pharmacological Considerations D
$38 REFRESHER COURSE OUTLINE Newborn anaesthesia: pharmacological considerations D. Ryan Cook MD Inhalation anaesthetics Several investigators have studied the age-related For the inhalation anaesthetics there are age-related cardiovascular effects of the potent inhalation differences in uptake and distribution, in anaes- anaesthetics at known multiples of MAC. At thetic requirements as reflected by differences in end-tidal concentrations of halothane bracketing MAC, in the effects on the four determinants of MAC, Lerman er al. 2 noted no difference in the cardiac output (preload, afterload, heart rate and incidence of hypotension or bradycardia in neonates contractility), and in the sensitivity of protective and in infants one to six months of age. Likewise, cardiovascular reflexes (e.g., baroreceptor reflex). we 3 have noted no age-related differences in the These differences limit the margin of safety of the determinants of cardiac output in developing piglets potent agents in infants. 1-20 days during either halothane or isoflurane Alveolar uptake of inhalation anaesthetics and anaesthesia. Halothane produced hypotension by a hence whole-body uptake is more rapid in infants reduction in contractility and heart rate; in much than in adults. J Lung washout is more rapid in older animals halothane also decreases peripheral infants than in adults because of the larger ratio vascular resistance. In piglets although blood pres- between alveolar minute ventilation and lung sure was equally depressed with isoflurane and volume (FRC) (3.5:1 in the infant vs. 1.3:1 in the halothane, cardiac output was better preserved adult). Increased brain size (ml.kg-J), limited during isoflurane anaesthesia. -
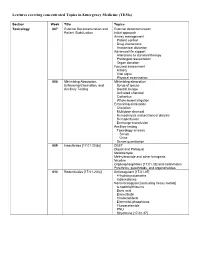
Lectures Covering Concentrated Topics in Emergency Medicine (Tems)
Lectures covering concentrated Topics in Emergency Medicine (TEMs) Section Week Title Topics Toxicology 007 External Decontamination and External decontamination Patient Stabilization Initial approach Airway management Patient control Drug interactions Anatomical distortion Advanced life support Alterations to standard therapy Prolonged resuscitation Organ donation Focused assessment History Vital signs Physical examination 008 Minimizing Absorption, Minimizing absorption Enhancing Elimination, and Syrup of ipecac Ancillary Testing Gastric lavage Activated charcoal Cathartics Whole-bowel irrigation Enhancing elimination Chelation Multidose charcoal Hemodialysis and peritoneal dialysis Hemoperfusion Exchange transfusion Ancillary testing Toxicology screens Serum Urine Serum quantitation 009 Insecticides [17.01.20(b)] DEET Diquat and Paraquat Metaldehyde Methylbromide and other fumigants Nicotine Organophosphates [17.01.33] and carbamates Pyrethrins, pyrethroids, and organohalides 010 Rodenticides [17.01.20(c)] Anticoagulant [17.01.05] 4-hydroxycoumarins Indanediones Nonanticoagulant [excluding heavy metals] α-naphthylthiourea Boric acid Bromethalin Cholecalciferol Elemental phosphorus Fluroacetamide PNU Strychnine [17.01.37] 011 Hydrocarbons [17.01.23] Aliphatic Aromatic General Toluene Xylene Halogenated General Carbon tetrachloride Methylene chloride Trichloroethylene Pine Oil and Turpentine Polychlorinated biphenyls and related substances 012 Caustic & Corrosive Agents Ingestions Acid [17.01.14(a)] Alkali [17.01.14(b)] Other Disc batteries