Blood Transfusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transfusion Service Introduction Blood/Blood Products Requests and Turnaround Time Expectations
Transfusion Service Introduction All blood products and blood components are supplied to UnityPoint Health-Meriter Hospital by the American Red Cross Blood Services. Pathology consultation is available regarding blood and/or components and dosages. ABO and Rho(D)—specific type is used whenever possible for leuko-poor packed cell transfusions. ABO-compatible blood is used for all plasma and platelet components whenever possible. For any orders involving HLA-matched components, the patient must have been HLA typed (sent to the American Red Cross) a minimum of 48 hours prior to intended infusion of the component. HLA typing is only required once. Blood components that are thawed, pooled, washed, volume-reduced, or deglycerolized for a patient will be charged to the patient even if not transfused. The charge is done because these components may not be suitable for another patient. Examples include: Autologous or directed donations Pooled cryoprecipitate 4-hour expiration Thawed fresh frozen plasma 24-hour expiration Thawed cryoprecipitate 6-hour expiration Deglycerolized red cells 24-hour expiration Washed red cells 24-hour expiration All blood components must be completely infused within 4 hours of release from UnityPoint Health-Meriter Laboratories Blood Bank, or be infused within the expiration time. Refer to UnityPoint Health -Meriter’s Blood and Blood Products Transfusion Policy #123 for additional information located on MyMeriter. Blood/Blood Products Requests and Turnaround Time Expectations Requests from UnityPoint Health-Meriter Hospital are entered in the hospital computer system and print in the UnityPoint Health- Meriter Laboratories Blood Bank. For the comfort of the patient, it is important to coordinate collection for other tests with Blood Bank specimens. -
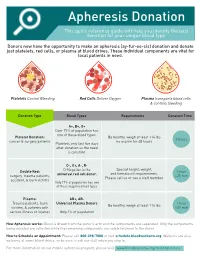
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Patient's Guide to Blood Transfusions
Health Information For Patients and the Community A Patient’s Guide to Blood Transfusions Your doctor may order a blood transfusion as part of your therapy. This brochure will focus on frequently asked questions about blood products, transfusions, and the risks and benefits of the blood transfusion. PLEASE NOTE: This information is not intended to replace the medical advice of your doctor or health care provider and is intended for educational purposes only. Individual circumstances will affect your individual risks and benefits. Please discuss any questions or concerns with your doctor. What is a blood transfusion? A blood transfusion is donated blood given to patients with abnormal blood levels. The patient may have abnormal blood levels due to blood loss from trauma or surgery, or as a result of certain medical problems. The transfusion is done with one or more of the following parts of blood: red blood cells, platelets, plasma, or cryoprecipitate. What are the potential benefits of a blood transfusion? If your body does not have enough of one of the components of blood, you may develop serious life-threatening complications. • Red blood cells carry oxygen through your body to your heart and brain. Adequate oxygen is very important to maintain life. • Platelets and cryoprecipitate help to prevent or control bleeding. • Plasma replaces blood volume and also may help to prevent or control bleeding. How safe are blood transfusions? Blood donors are asked many questions about their health, behavior, and travel history in order to ensure that the blood supply is as safe as it can be. Only people who pass the survey are allowed to donate. -

Patient Information Leaflet – Plasma Exchange Procedure
Therapeutic Apheresis Services Patient Information Leaflet – Plasma Exchange Procedure Introduction Antibodies, which normally help to protect you from infection, can begin to attack your own This leaflet has been written to give patients healthy cells, or an over production of proteins can information about plasma exchange (sometimes cause your blood to become thicker and slow down called plasmapheresis). If you would like any more the blood flow throughout your body. A plasma information or have any questions, please ask the exchange can help improve your symptoms, doctors and nurses involved in your treatment at although this may not happen immediately. the NHS Blood and Transplant (NHSBT) Therapeutic Apheresis Services Unit. Although plasma exchange may help with symptoms, it will not normally cure your condition When you have considered the information given as it does not switch off the production of the in this leaflet, and after we have discussed the harmful antibodies or proteins. It is likely that procedure and its possible risks with you, we will this procedure will form only one part of your ask you to sign a consent form to indicate that you treatment. are happy for the procedure to go ahead. Before any further procedures we will again check that you are happy to proceed. How do we perform Plasma Exchange? What is a plasma exchange? Plasma exchange is performed using a machine Blood is made up of red cells, white cells and called a Blood Cell Separator which can separate platelets which are carried around in fluid called blood into its various parts. The machine separates plasma. -

Your Blood Cells
Page 1 of 2 Original Date The Johns Hopkins Hospital Patient Information 12/00 Oncology ReviseD/ RevieweD 6/14 Your Blood Cells Where are Blood cells are made in the bone marrow. The bone marrow blood cells is a liquid that looks like blood. It is found in several places of made? the body, such as your hips, chest bone and the middle part of your arm and leg bones. What types of • The three main types of blood cells are the red blood cells, blood cells do the white blood cells and the platelets. I have? • Red blood cells carry oxygen to all parts of the body. The normal hematocrit (or percentage of red blood cells in the blood) is 41-53%. Anemia means low red blood cells. • White blood cells fight infection. The normal white blood cell count is 4.5-11 (K/cu mm). The most important white blood cell to fight infection is the neutrophil. Forty to seventy percent (40-70%) of your white blood cells should be neutrophils. Neutropenia means your neutrophils are low, or less than 40%. • Platelets help your blood to clot and stop bleeding. The normal platelet count is 150-350 (K/cu mm). Thrombocytopenia means low platelets. How do you Your blood cells are measured by a test called the Complete measure my Blood Count (CBC) or Heme 8/Diff. You may want to keep track blood cells? of your blood counts on the back of this sheet. What When your blood counts are low, you may become anemic, get happens infections and bleed or bruise easier. -

Important Information for Female Platelet Donors
Important Information for Female Platelet Donors We are grateful for the support you provide our community blood program and especially appreciate your willingness to help save lives as a volunteer platelet donor. We also take our responsibility to provide a safe and adequate blood supply very seriously and need to share the following information regarding a change to our donor eligibility criteria for female platelet donors. We recently began performing a Human Leukocyte Antigen (HLA) antibody test on each of our current female platelet donors who have ever been pregnant. In addition, we modified our Medical History Questionnaire to ask donors whether they have been pregnant since their last donation. Platelet donors who respond yes to that question will be screened for HLA antibodies. Platelet donors will also be retested after every subsequent pregnancy. These adjustments are being made as part of our effort to reduce occurrences of Transfusion-Related Acute Lung Injury (TRALI). TRALI is a rare but serious complication of blood transfusions most commonly thought to be caused by a reaction to HLA antibodies present in the donor’s plasma. When transfused, these antibodies can sometimes cause plasma to leak into the patient’s lungs, creating fluid accumulation — a condition referred to as acute pulmonary edema. Female donors who have been pregnant are more likely than others to have these HLA antibodies in their plasma. Once the antibodies develop, they are present forever. The antibodies could be harmful if transfused into certain patients. The antibodies are present in plasma — and platelet donations contain a high volume of plasma, so our current efforts are directed at screening blood samples from female platelet donors to test for the HLA antibody. -

FACTS ABOUT DONATING Blood WHY SHOULD I DONATE BLOOD? HOW MUCH BLOOD DO I HAVE? Blood Donors Save Lives
FACTS ABOUT DONATING BLOOD WHY SHOULD I DONATE BLOOD? HOW MUCH BLOOD DO I HAVE? Blood donors save lives. Volunteer blood donors provide An adult has about 10–11 pints. 100 percent of our community’s blood supply. Almost all of us will need blood products. HOW MUCH BLOOD WILL I donate? Whole blood donors give 500 milliliters, about one pint. WHO CAN DONATE BLOOD? Generally, you are eligible to donate if you are 16 years of WHAT HAPPENS TO BLOOD AFTER I donate? age or older, weigh at least 110 pounds and are in good Your blood is tested, separated into components, then health. Donors under 18 must have written permission distributed to local hospitals and trauma centers for from a parent or guardian prior to donation. patient transfusions. DOES IT HURT TO GIVE BLOOD? WHAT ARE THE MAIN BLOOD COMPONENTS? The sensation you feel is similar to a slight pinch on the Frequently transfused components include red blood arm. The process of drawing blood should take less than cells, which replace blood loss in patients during surgery ten minutes. or trauma; platelets, which are often used to control or prevent bleeding in surgery and trauma patients; and HOW DO I PREPARE FOR A BLOOD DONATION? plasma, which helps stop bleeding and can be used to We recommend that donors be well rested, eat a healthy treat severe burns. Both red blood cells and platelets meal, drink plenty of fluids and avoid caffeine and are often used to support patients undergoing cancer alcohol prior to donating. treatments. WHAT CAN I EXPECT WHEN I DONATE? WHEN CAN I DONATE AGAIN? At every donation, you will fill out a short health history You can donate whole blood every eight weeks, platelets questionaire. -

FDA Regulation of Blood and Blood Components in the United States
FDA Regulation of Blood and Blood Components in the United States SLIDE 1 This presentation will review the FDA Regulation of Blood and Blood Components in the U.S. SLIDE 2 The FDA Center for Biologics Evaluation and Research, or CBER, Office of Blood Research and Review, called OBRR, reviews several different types of regulatory applications with respect to blood and blood components. This includes biologics license applications, called BLAs, which represent the regulatory pathway for blood components. The BLA regulatory process also applies to biological drugs such as fractionated plasma products. OBRR also regulates in-vitro diagnostic devices used for screening of collected blood, and does so also using the BLA regulatory pathway. Review of these devices as biologic licenses allows CBER to apply a higher level of manufacturing oversight, including lot release testing and pre-licensure inspection. This regulatory pathway applies to infectious disease tests for blood screening, as well as blood grouping and phenotyping reagents. SLIDE 3 The regulatory pathway for New Drug Applications, or NDAs, is most commonly used within the Center for Drug Evaluation and Research, or CDER. Within the Office of Blood, several NDA applications are reviewed each year, mostly involving solutions used for the collection of blood, such as anticoagulants and red cell nutritive solutions. Interestingly, a blood bag that does not have a solution inside is regulated as a device. If the bag has a solution, then it is a drug-device combination product, but is regulated as a drug. SLIDE 4 OBRR reviews both Class Two and Class Three devices. Class Three devices in OBRR include diagnostic tests for HIV regulated as pre-market approvals, called PMAs. -

Terminology Resource File
Terminology Resource File Version 2 July 2012 1 Terminology Resource File This resource file has been compiled and designed by the Northern Assistant Transfusion Practitioner group which was formed in 2008 and who later identified the need for such a file. This resource file is aimed at Assistant Transfusion Practitioners to help them understand the medical terminology and its relevance which they may encounter in the patient’s medical and nursing notes. The resource file will not include all medical complaints or illnesses but will incorporate those which will need to be considered and appreciated if a blood component was to be administered. The authors have taken great care to ensure that the information contained in this document is accurate and up to date. Authors: Jackie Cawthray Carron Fogg Julia Llewellyn Gillian McAnaney Lorna Panter Marsha Whittam Edited by: Denise Watson Document administrator: Janice Robertson ACKNOWLEDGMENTS We would like to acknowledge the following people for providing their valuable feedback on this first edition: Tony Davies Transfusion Liaison Practitioner Rose Gill Transfusion Practitioner Marie Green Transfusion Practitioner Tina Ivel Transfusion Practitioner Terry Perry Transfusion Specialist Janet Ryan Transfusion Practitioner Dr. Hazel Tinegate Consultant Haematologist Reviewed July 2012 Next review due July 2013 Version 2 July 2012 2 Contents Page no. Abbreviation list 6 Abdominal Aortic Aneurysm (AAA) 7 Acidosis 7 Activated Partial Thromboplastin Time (APTT) 7 Acquired Immune Deficiency Syndrome -

Human ICAM-4 Antibody
Human ICAM-4 Antibody Monoclonal Mouse IgG2B Clone # 729632 Catalog Number: MAB8397 DESCRIPTION Species Reactivity Human Specificity Detects human ICAM4 in direct ELISAs. Source Monoclonal Mouse IgG2B Clone # 729632 Purification Protein A or G purified from hybridoma culture supernatant Immunogen Chinese hamster ovary cell line CHOderived recombinant human ICAM4 Ala23Ala240 Accession # Q14773 Formulation Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. See Certificate of Analysis for details. *Small pack size (SP) is supplied either lyophilized or as a 0.2 μm filtered solution in PBS. APPLICATIONS Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website. Recommended Sample Concentration Flow Cytometry 0.25 µg/106 cells See Below CyTOFready Ready to be labeled using established conjugation methods. No BSA or other carrier proteins that could interfere with conjugation. DATA Flow Cytometry Detection of ICAM4 in Human Red Blood Cells by Flow Cytometry. Human red blood cells were stained with Mouse Anti Human ICAM4 Monoclonal Antibody (Catalog # MAB8397, filled histogram) or isotype control antibody (Catalog # MAB0041, open histogram), followed by Allophycocyaninconjugated AntiMouse IgG Secondary Antibody (Catalog # F0101B). PREPARATION AND STORAGE Reconstitution Reconstitute at 0.5 mg/mL in sterile PBS. Shipping The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. *Small pack size (SP) is shipped with polar packs. Upon receipt, store it immediately at 20 to 70 °C Stability & Storage Use a manual defrost freezer and avoid repeated freezethaw cycles. -
Laboratory Best Transfusion Practice for Neonates, Infants and Children
Laboratory Best Transfusion Practice for Neonates, Infants and Children This summary guidance should be used in conjunction with the appropriate 20161 and 20122 BSH Guidelines and laboratory SOPs Compatibility testing Neonates and infants < 4 months Obtain neonatal and maternal transfusion history (including any fetal transfusions) for all admissions. Obtain a maternal sample for initial testing where possible, in addition to the patient sample. Red cell selection: no maternal antibodies present Select appropriate group and correct neonatal specification red cells. Group O D-negative red cells may be issued electronically without serological crossmatch. If the laboratory does not universally select group O D-negative red cells for this age group, blood group selection should either be controlled by the LIMS or an IAT crossmatch should be performed using maternal or neonatal plasma to serologically confirm ABO compatibility with both mother and neonate. Red cell selection: where there is maternal antibody Select appropriate group red cells, compatible with maternal alloantibody/ies. An IAT crossmatch should be performed using the maternal plasma. If it is not possible to obtain a maternal sample it is acceptable to crossmatch antigen-negative units against the infant’s plasma. Where paedipacks are being issued from one donor unit it is only necessary to crossmatch the first split pack. Subsequent split packs from this multi-satellite unit can be automatically issued without further crossmatch until the unit expires or the infant is older than 4 months. If packs from a different donor are required, an IAT crossmatch should be performed. Infants and children ≥ 4 months For infants and children from 4 months of age, pre-transfusion testing and compatibility procedures should be performed as recommended for adults. -

Understanding the Immune System: How It Works
Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute NIH Publication No. 03-5423 September 2003 www.niaid.nih.gov www.nci.nih.gov Contents 1 Introduction 2 Self and Nonself 3 The Structure of the Immune System 7 Immune Cells and Their Products 19 Mounting an Immune Response 24 Immunity: Natural and Acquired 28 Disorders of the Immune System 34 Immunology and Transplants 36 Immunity and Cancer 39 The Immune System and the Nervous System 40 Frontiers in Immunology 45 Summary 47 Glossary Introduction he immune system is a network of Tcells, tissues*, and organs that work together to defend the body against attacks by “foreign” invaders. These are primarily microbes (germs)—tiny, infection-causing Bacteria: organisms such as bacteria, viruses, streptococci parasites, and fungi. Because the human body provides an ideal environment for many microbes, they try to break in. It is the immune system’s job to keep them out or, failing that, to seek out and destroy them. Virus: When the immune system hits the wrong herpes virus target or is crippled, however, it can unleash a torrent of diseases, including allergy, arthritis, or AIDS. The immune system is amazingly complex. It can recognize and remember millions of Parasite: different enemies, and it can produce schistosome secretions and cells to match up with and wipe out each one of them.