Knoevenagel Condensation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alkyl Halides Work Best; Secondary Alkyl Halides Give Lower Yields
CHAPTER 21 ESTER ENOLATES ou have already had considerable experience with carbanionic compounds and their applications in synthetic organic chemistry. The first was acetylide ion in YChapter 9, followed in Chapter 14 by organometallic compounds—Grignard reagents, for example—that act as sources of negatively polarized carbon. In Chapter 18 you learned that enolate ions—reactive intermediates generated from aldehydes and ketones—are nucleophilic, and that this property can be used to advantage as a method for carbon–carbon bond formation. The present chapter extends our study of carbanions to the enolate ions derived from esters. Ester enolates are important reagents in synthetic organic chemistry. The stabilized enolates derived from -keto esters are particularly useful. OO C ␣ C  R CH2 ORЈ -Keto ester: a ketone carbonyl is  to the carbonyl group of the ester. A proton attached to the ␣-carbon atom of a -keto ester is relatively acidic. Typical Ϫ11 acid dissociation constants Ka for -keto esters are Ϸ10 (pKa 11). Because the ␣- carbon atom is flanked by two electron-withdrawing carbonyl groups, a carbanion formed at this site is highly stabilized. The electron delocalization in the anion of a -keto ester is represented by the resonance structures 831 832 CHAPTER TWENTY-ONE Ester Enolates Ϫ Ϫ O O O O O O C C C C C C R C ORЈ R ϪC ORЈ R C ORЈ H H H Principal resonance structures of the anion of a -keto ester We’ll begin by describing the preparation and properties of -keto esters, proceed to a discussion of their synthetic applications, continue to an examination of related species, and conclude by exploring some recent developments in the active field of synthetic car- banion chemistry. -

Knoevenagel Condensation Reaction Catalysed by Agro-Waste Extract As a Greener Solvent Catalyst
ORIGINAL ARTICLE Org. Commun. 14:1 (2021) 81-91 Knoevenagel condensation reaction catalysed by agro-waste extract as a greener solvent catalyst Krishnappa B. Badiger and Kantharaju Kamanna * Peptide and Medicinal Chemistry Research Laboratory, Department of Chemistry, Rani Channamma University, P-B, NH-4, Belagavi 591 156, India (Received January 22, 2021; Revised March 15, 2021; Accepted March 18, 2021) Abstract: This paper present a novel Knoevenagel reaction protocol for the condensation of aromatic/heteroaromatic aldehydes with malononitrile to give α, β–unsaturated benzylidene derivatives. The main focus of this work is to reveal the usability of agro-waste extracts as a catalyst in the Knoevenagel condensation. The present protocol proceeds efficiently for various substituted aromatic and heterocyclic aldehydes in the Knoevenagel reactions. In addition, the present method describes direct isolation of the formed products without using organic solvent extraction gave good yields product. Keywords: one-pot reaction; green chemistry; solvent-free; Knoevenagel condensation reaction; malononitrile; room temperature. ©2021 ACG Publication. All right reserved. 1. Introduction The carbon-carbon bond formation via Knoevenagel condensation1-3 is one of the most important routes repeated in the synthetic organic chemistry and allows the production of various active pharmaceutical molecules.4 Figure 1. Structure of biologically potent benzylidinemalononitrile derivatives * Corresponding author:E-Mail: [email protected] The article was published -

Microwave-Assisted Urea Catalyzed Knoevenagel Condensation of Aldehydes with = Light Brown Solid, M.P
Available online at www.banglajol.info Bangladesh J. Sci. Ind. Res. 55(2), 159-164, 2020 purification. The Chemicals 4-fluorobenzaldehyde (98%), 2-(4-nitrophenylmethylene)malononitrile (3a): yield 90%, 2-(4-Hydroxyphenylmethylene)malononitrile (3f): yield CDCl3) δ(ppm): 8.32(s, 1H), 8.01(d, J=8.4, 2H), 7.22(d, Furthermore, the treatment of aldehyde such as acknowledge the University Research Centre, SUST for Yang Y, Dong ODW, Pan W, Zhang J and Liu Q (2006), Wang C, Li Guishen S, Li G, Feng J, Li S and Xiaolu (2001), 4-hydroxybenzaldehyde (98%), 4-nitrobenzaldehyde yellowish white solid, m.p. 158-159 , (lit.160 (Sun Qi et 96%, yellow solid, m.p. 171-172 , (lit. 189-190 ( Sun J=8.4, 2H), 6.38(s, 1H, N-H), 6.11(s, 1H, N-H) . cinnamaldehyde with ethylcyanoacetate also give financial support. Facile and clean synthesis of α-alkenoyl Synthesis of 5-alkylene barbituric acid in solventless 2 Qi et al 2005 ); IR (KBr): υ (cm-1): 3354(-OH), olefinic compounds 3i under similar conditions (Table ketene-(S,S)-acetal via the aldol condensation under microwave irradiation, Chin. J. Org. Chem. (98%), 2-nitrobenzaldehyde (98%), 3-nitrobenzaldehyde al 2005)); IR (KBr): υmax: 3039(sp C-H), 2231 (C≡N), 1604 max (98%), 4-chlorobenzaldehyde (97%) and cyanoacetamide (C=C) and 1521 and 1344(N-O) cm℃-1; 1H NMR℃ (400 MHz, 3030(sp2 C-H), 2227(C≡N), and℃ 1670(C=C) cm℃-1; 1H 2-cyano-3-(4-nitrophenyl)acrylamide (3l): yield 85%, 1, Scheme 1). Both electron-rich and References reactions in water, Tetrahedron 62: 10-111. -

Green Chemistry – Aspects for the Knoevenagel Reaction
2 Green Chemistry – Aspects for the Knoevenagel Reaction Ricardo Menegatti Universidade Federal de Goiás Brazil 1. Introduction Knoevenagel condensation is a classic C-C bond formation reaction in organic chemistry (Laue & Plagens, 2005). These condensations occur between aldehydes or ketones and active methylene compounds with ammonia or another amine as a catalyst in organic solvents (Knoevenagel, 1894). The Knoevenagel reaction is considered to be a modification of the aldol reaction; the main difference between these approaches is the higher acidity of the active methylene hydrogen when compared to an -carbonyl hydrogen (Smith & March, 2001). Figure 1 illustrates the condensation of a ketone (1) with a malonate compound (2) to form the Knoevenagel condensation product (3), which is then used to form the ,-unsaturated carboxylic compounds (3) and (4) (Laue & Plagens, 2005). R R R O O O O H O O O + base hydrolysis H O O O O R R (1) (2) (3) (4) Fig. 1. An example of the Knoevenagel reaction. Subsequent to the first description of the Knoevenagel reaction, changes were introduced using pyridine as the solvent and piperidine as the catalyst, which was named the Doebner Modification (Doebner, 1900). The Henry reaction is another variation of the Knoevenagel condensation that utilises compounds with an -nitro active methylene (Henry, 1895). The general mechanism for the Knoevenagel reaction, which involves deprotonation of the malonate derivative (6) by piperidine (5) and attack by the formed carbanion (8) on the carbonyl subunit (9) as an aldol reaction that forms the product (10) of the addition step is illustrated in Fig. -
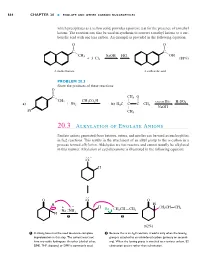
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Experiment 2: Two Step Synthesis of Ethyl 4-Methoxycinnamate
Experiment 2: Two step synthesis of ethyl 4-methoxycinnamate Background Why is ethyl 4-methoxycinnamate (1) interesting? First, it is the analog of octyl 4-methoxycinnamate (2), a UV light blocker found in Bain de Soleil All Day Sunblock and Coppertone Sport. The derivatives of 4-methoxycinnamic acid absorb UVB radiation due to their high level of conjugation. They are also oil soluble. UVB radiation promotes dermal cell DNA damage causing skin cancer. Octyl 4-methoxycinnamate is used in sunscreen over ethyl 4-methoxycinnamate because of its higher molar absorptivity ε and its greater lipophilicity. O R O O R = -CH3CH3 ethyl 4-methoxycinnamate (1) R = octyl 4-methoxycinnamate (2) Figure 1: Derivatives of 4-methoxycinnamic acid Second, a two step synthesis is put forth so that you can practice multi-step synthesis using a variety of laboratory techniques before diving into Honors Cup. Step 1 is the Verley-Doebner modification of the Knoevenagel condensation.1 The Knoevenagel condensation reaction dates from 1896 and is a facile one-step method of forming new carbon- carbon bonds under mild reaction conditions. It utilizes a weak amine base such as piperidine to initially form an enolate anion derived from a 1,3-dicarbonyl compound. The enolate subsequently acts as a nucleophile in the condensation with another carbonyl compound. This carbonyl is often an aldehyde and the reaction mechanism bears striking similarity to that of the aldol condensation. The isolated product is generally an α,β-conjugated carboxylic acid, formed via ready dehydration of the intermediate β-hydroxydicarbonyl compound and subsequent saponification and decarboxylation. -

Route of Knoevenagel Reaction from Conventional Method to Greener Methods
IOSR Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT) e-ISSN: 2319-2402,p- ISSN: 2319-2399.Volume 9, Issue 12 Ver. I (Dec. 2015), PP 52-55 www.iosrjournals.org Route of Knoevenagel Reaction from Conventional method to Greener methods Dr. Leena H. Sarkar Department of Chemistry, J.V.M’s Degree College, Airoli, Navi Mumbai 400 708, India. Abstract: In the past few years, classic methods of synthesis have been replaced by newer methods of synthesis involving principles of Green Chemistry given by Anastas and Warner leading to environmentally safer products and processes and reducing pollution and thus making the environment greener. In this review article I have compared old classical method of Knoevenagel reaction with greener methods for a more sustainable, pollution free future society. Key Words: Green chemistry, Knoevenagel reaction, solvent free reactions, sustainable chemistry, use of ultrasound radiations, catalysis, microwave chemistry and use of ionic liquids. I. Introduction The Knoevenagel [1] reaction is considered to be a modification of the aldol reaction [2]. It is named after Emil Knoevenagel. It is a condensation reaction of aldehydes or ketones 1 with active methylene compounds 2 in presence of base like ammonia or another amine or piperidine as a catalyst in organic solvents to yield α,β-unsaturated carbonyl compounds 3 (Fig. 1) which serve as intermediates in various reactions and compounds of medicinal interest [3-7]. Group attached to methylene group must be powerful enough to facilitate deprotonation to form enolate ion, even with a mild base. Fig. 1: Conventional method of Knoevenagel synthesis II. -

DABCO-Catalyzed Knoevenagel Condensation of Aldehydes with Ethyl Cyanoacetate Using Hydroxy Cite This: RSC Adv.,2018,8,30180 Ionic Liquid As a Promoter†
RSC Advances View Article Online PAPER View Journal | View Issue DABCO-catalyzed Knoevenagel condensation of aldehydes with ethyl cyanoacetate using hydroxy Cite this: RSC Adv.,2018,8,30180 ionic liquid as a promoter† Dan Meng,a Yongsheng Qiao,b Xin Wang,b Wei Wenb and Sanhu Zhao *ab N-(2-Hydroxy-ethyl)-pyridinium chloride ([HyEtPy]Cl) was synthesized and explored as a novel promoter for 1,4-diazabicyclo [2.2.2] octane (DABCO)-catalyzed Knoevenagel condensation reactions, which showed better catalytic activity compared to other ionic liquid (IL) that had no hydroxyl group attached to the IL scaffold. The effect of hydrogen bond formation between the hydroxyl group of [HyEtPy]Cl and the carbonyl group of aldehyde played an important role in the Knoevenagel condensation reaction. In the [HyEtPy]Cl–H2O–DABCO composite system, Knoevenagel condensation reactions proceeded Received 2nd August 2018 smoothly and cleanly, and the corresponding Knoevenagel condensation products were obtained in Accepted 14th August 2018 good to excellent yields in all cases examined. This protocol provides a versatile solvent–catalyst system, DOI: 10.1039/c8ra06506c Creative Commons Attribution-NonCommercial 3.0 Unported Licence. which has notable advantages such as being eco-friendly, ease of work-up and convenient reuse of the rsc.li/rsc-advances ionic liquid. Introduction Currently, ionic liquids (ILs) are receiving great attention for their application as innovative solvents or additives in a variety The Knoevenagel reaction, which was discovered by Knoeve- of organic reactions.19 In comparison to the common molecular nagel in 1896, is a condensation reaction between activated solvents, the main characteristic of ILs is that they are methylene and carbonyl compounds.1 Owing to the fact that the completely composed of ions, which makes them ideal candi- 20 This article is licensed under a a,b-unsaturated carbonyl compounds produced by Knoevenagel dates to stabilize the intermediate of the addition reaction. -

Solvent-Free and Aqueous Knoevenagel Condensation of Aromatic Ketones with Malononitrile
Issue in Honor of Prof. Cheng-Ye Yuan ARKIVOC 2004 (ix) 4-8 Solvent-free and aqueous Knoevenagel condensation of aromatic ketones with malononitrile Guan-Wu Wang* and Bo Cheng Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China E-mail: [email protected] Dedicated to Professor Chengye Yuan on his 80th birthday (received 28 Feb 04; accepted 26 Apr 04; published on the web 01 May 04) Abstract The microwave irradiation-assisted and thermal solvent-free Knoevenagel condensations of aromatic ketones with malononitrile catalyzed by NH4OAc or silica gel, and the uncatalyzed Knoevenagel condensations in refluxing water have been investigated. Keywords: Knoevenagel condensation, solvent-free, aqueous, aromatic ketone, microwave irradiation, thermal heating Introduction Organic reactions under solvent-free1,2 and aqueous3,4,5 conditions have increasingly attracted chemists’ interests, particularly from the viewpoint of green chemistry.6 As an important carbon- carbon bond forming reaction, Knoevenagel condensation has been extensively studied. Generally, this type of reaction is catalyzed by base or Lewis acid in the liquid-phase system. In recent years, chemists paid more and more attention to the clean synthesis of alkenes by Knoevenagel condensations. The Knoevenagel condensations between aldehydes and 7 8 malononitrile in dry media catalyzed by ZnCl2, silica gel and ammonium acetate (NH4OAc)- basic alumina9 have been reported. Recently Bigi’s group described the same reactions which could proceed efficiently in water without any catalyst.10 More recently, we found that aldehydes reacted with malononitrile efficiently in the absence of catalyst and solvent under microwave irradiation and thermal heating conditions.11 However, much less work on the Knoevenagel condensation involving ketones has been done because ketones are generally very unreactive towards Knovenagel condensation. -

Use of Piperidine and Pyrrolidine in Knoevenagel Condensation
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Research Article Organic & Medicinal Chem IJ Volume 5 Issue 3 - March 2018 Copyright © All rights are reserved by Mauri Sergio Alves Palma DOI: 10.19080/OMCIJ.2018.05.555668 Use of Piperidine and Pyrrolidine in Knoevenagel Condensation Rodrigo De Oliveira Vieira, Edson Nascimento Dos Santos, Gabriela Consolini and Mauri Sergio Alves Palma* Department of Pharmaceutical and Biochemical Technology, University of São Paulo, Brazil Submission: February 15, 2018; Published: March 01, 2018 *Corresponding author: Mauri Sergio Alves Palma, Department of Pharmaceutical and Biochemical Technology, University of São Paulo, 580, Professor Lineu Prestes Avenue, São Paulo, Brazil, Tel: , Email: Abstract Glitazones are an important class of drugs used specially in the treatment of diabetes mellitus type II. Pharmaceuticals intermediates which base as catalyst. In this study, tiazolidine-2,4-dione (TZD) was reacted with p-metoxybenzaldehyde and p-nitrobenzaldehyde, using piperidine originates glitazones can be formed by the reaction of a carbonyl with an activated methylene, though Knoevenagel condesantion using a weak Pyrrolidine showed higher value of TZD conversion than piperidine for both aldehydes, reaching a conversion of 100% to 4-methoxybenzaldehyde withand pyrrolidine lower amount as catalysts. of pyrrolidine The influence corresponding of the tosubstituent 62.5% of piperidine.was evident, 4-metoxybenzaldehyde showed the highest value of TZD conversion. Keywords: Organic synthesis; Thiazolidine-2,4-dione; Pharmaceutical derivatives Abbreviations: TZD: Tiazolidine-2,4-Dione; PPARϒ: Peroxisome Proliferator-Activated Receptor Gamma; TNF: Tumour Necrosis Factor; iNOS: induced Nitric Oxide Synthase; ANVISA: National Agency for Sanitary Vigilance Introduction lipids, leading to diabetes. Therefore, TZD derivatives act in this Currently, the number of cases of people with diabetes in Brazil is approximately 14 million, according to the International antioxidant activity. -

Solvent-Free Selective Condensations Based on the Formation of the Olefinic
catalysts Article Solvent-Free Selective Condensations Based on the Formation of the Olefinic (C=C) Bond Catalyzed by Organocatalyst Heyuan Song 1,2, Ronghua Jin 1, Fuxiang Jin 1, Meirong Kang 1, Zhen Li 1,* and Jing Chen 1,* 1 State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China; [email protected] (H.S.); [email protected] (R.J.); [email protected] (F.J.); [email protected] (M.K.) 2 Graduate school, University of Chinese Academy of Sciences, Beijing 100049, China * Correspondence: [email protected] (Z.L.); [email protected] (J.C.); Tel.: +86-931-496-8056 (Z.L.); +86-931-496-8068 (J.C.); Fax: +86-931-496-8129 (Z.L. & J.C.) Academic Editors: Aurelio G. Csákÿ and Keith Hohn Received: 6 June 2016; Accepted: 14 July 2016; Published: 20 July 2016 Abstract: Pyrrolidine and its derivatives were used to catalyze aldol and Knoevenagel condensations for the formation of the olefinic (C=C) bond under solvent-free conditions. The 3-pyrrolidinamine showed high activity and afforded excellent yields of α,β-unsaturated compounds. The aldol condensation of aromatic/heterocyclic aldehydes with ketones affords enones in high conversion (99.5%) and selectivity (92.7%). Good to excellent yields of α,β-unsaturated compounds were obtained in the Knoevenagel condensation of aldehydes with methylene-activated substrates. Keywords: aldol condensation; Knoevenagel condensation; organocatalysis; solvent-free condition; ketone; aldehyde 1. Introduction The formation of a new olefinic (C=C) bond, which is one of the most fundamental transformations in organic synthesis, is well represented by aldol and Knoevenagel condensations. -

Carboxylic Acids and Their Derivatives
8 CARBOXY A lmost all of the basic types of reactions now have been covered: addition, elimination, substitution, and rearrangement by polar, radical, and concerted mechanisms. Indeed, if you have been looking for similarities, you will have seen that most of the reactions discussed in the preceding three chapters are variations on basic types we have discussed earlier. Furthermore, most of the basic structural effects that determine chemical reactivity also have been covered in previous chapters: bond energies, steric hindrance, electronega- tivity, electron delocalization, hydrogen bonding, solvation, and conforma- tional influences. You might well ask what is left. The answer is, a great deal- but now we will be concerned mostly with putting concepts together, moving from the simple to the complex. For example, in this chapter we will be trying to under- stand the ways that carboxylic acids, which possess the -c' functional \ group, are similar to and different from alcohols, which have the OH group, and aldehydes and ketones, which have C=O bonds. Subsequently we will look at acids that also possess OH or NH, sub- stituent groups (or both) and develop a rationale for the behavior of these combinations in terms of effects we already have discussed. Insofar as pos- sible, you should try to do this yourself whenever you encounter a substance with a new set of combinations of functional groups on its molecules. You often will be in error (as many experts will be), because even if you take Carboxylic Acids and Their Derivatives 789 account of all of the structural effects, as well as the possible reactions or inter- actions, the overall result of these frequently is very difficult to judge in advance.