Carboxylic Acids and Their Derivatives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alkyl Halides Work Best; Secondary Alkyl Halides Give Lower Yields
CHAPTER 21 ESTER ENOLATES ou have already had considerable experience with carbanionic compounds and their applications in synthetic organic chemistry. The first was acetylide ion in YChapter 9, followed in Chapter 14 by organometallic compounds—Grignard reagents, for example—that act as sources of negatively polarized carbon. In Chapter 18 you learned that enolate ions—reactive intermediates generated from aldehydes and ketones—are nucleophilic, and that this property can be used to advantage as a method for carbon–carbon bond formation. The present chapter extends our study of carbanions to the enolate ions derived from esters. Ester enolates are important reagents in synthetic organic chemistry. The stabilized enolates derived from -keto esters are particularly useful. OO C ␣ C  R CH2 ORЈ -Keto ester: a ketone carbonyl is  to the carbonyl group of the ester. A proton attached to the ␣-carbon atom of a -keto ester is relatively acidic. Typical Ϫ11 acid dissociation constants Ka for -keto esters are Ϸ10 (pKa 11). Because the ␣- carbon atom is flanked by two electron-withdrawing carbonyl groups, a carbanion formed at this site is highly stabilized. The electron delocalization in the anion of a -keto ester is represented by the resonance structures 831 832 CHAPTER TWENTY-ONE Ester Enolates Ϫ Ϫ O O O O O O C C C C C C R C ORЈ R ϪC ORЈ R C ORЈ H H H Principal resonance structures of the anion of a -keto ester We’ll begin by describing the preparation and properties of -keto esters, proceed to a discussion of their synthetic applications, continue to an examination of related species, and conclude by exploring some recent developments in the active field of synthetic car- banion chemistry. -

Knoevenagel Condensation
Knoevenagel condensation The Knoevenagel condensation (pronounced [ˈknøːvənaːɡl̩ ]) Knoevenagel condensation reaction is an organic reaction named after Emil Knoevenagel. It is a Named after Emil Knoevenagel modification of the aldol condensation.[1][2] Reaction type Coupling reaction A Knoevenagel condensation is a nucleophilic addition of an active Identifiers hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence Organic knoevenagel- condensation). The product is often an α,β-unsaturated ketone (a Chemistry condensation conjugated enone). Portal RSC ontology RXNO:0000044 ID In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form[3] Z–CH2-Z or Z–CHR–Z for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid.[4] Z–CHR1R2 for instance nitromethane. where Z is an electron withdrawing functional group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldehyde or ketone. The Hantzsch pyridine synthesis, the Gewald reaction and the Feist–Benary furan synthesis all contain a Knoevenagel reaction step. The reaction also led to the discovery of CS gas. Contents Doebner modification Scope Weiss–Cook reaction See also References Doebner modification The Doebner modification of the Knoevenagel condensation. Acrolein and malonic acid react in pyridine to give trans-2,4-pentadienoic acid with the loss of carbon dioxide. With malonic compounds the reaction product can lose a molecule of carbon dioxide in a subsequent step. -
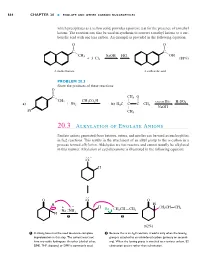
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Cyclopentane Synthesis
Cyclopentane Synthesis Dan O’Malley Baran Group Meeting Cyclopentane Synthesis Group Meeting O'Malley 2/9/2005 This presentation is broken down into the following catagories. Some reactions either fit more than one Students of organic chemistry are taught a number of reactions for the synthesis of category or do not fit easily into any of them. Efforts have been made to place all such reactions in the cyclohexanes at a very early stage of their careers. Techniques for the creation of cyclopentanes, most appropriate category. however, are generally taught at a much later stage and are rarely given the same detailed treatment. This may be the result of the fact that there are no equivalents of reactions such as the Diels-Alder and I. General Information Robinson Annulation in terms of generality, extent of use, and historical importance. This may, in turn, II. Ionic Reactions be caused by the fact that the cyclopentane is an inherintly "umpoled" functionality, as illustrated below. III. Metal Mediated Reactions IV. Radical Reactions FG V. Pericyclic and Pseudo-pericyclic Reactions VI. Ring Expansion and Contraction Reactions I. General Information This situation is further exacerbated by the general lack of cheaply available cyclopentane compounds Baldwin's rules in the chiral pool; wheras a number of cyclohexane terpenes are readily available for elaboration, there Baldwin has divided ring closure reactions into those that are "favored" and those that are "disfavored". are no analogous cylcopentane natural products. Cyclopentanes are however, present in many Those that are disfavored are not always impossible, but are frequently much more difficult to effect. -

Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy Petr Klan,́*,†,‡ Tomaś̌solomek,̌ †,‡ Christian G
Review pubs.acs.org/CR Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy Petr Klan,́*,†,‡ Tomaś̌Solomek,̌ †,‡ Christian G. Bochet,§ Aurelień Blanc,∥ Richard Givens,⊥ Marina Rubina,⊥ Vladimir Popik,# Alexey Kostikov,# and Jakob Wirz∇ † Department of Chemistry, Faculty of Science, Masaryk University, Kamenice 5, 625 00 Brno, Czech Republic ‡ Research Centre for Toxic Compounds in the Environment, Faculty of Science, Masaryk University, Kamenice 3, 625 00 Brno, Czech Republic § Department of Chemistry, University of Fribourg, Chemin du Museé 9, CH-1700 Fribourg, Switzerland ∥ Institut de Chimie, University of Strasbourg, 4 rue Blaise Pascal, 67000 Strasbourg, France ⊥ Department of Chemistry, University of Kansas, 1251 Wescoe Hall Drive, 5010 Malott Hall, Lawrence, Kansas 66045, United States # Department of Chemistry, University of Georgia, Athens, Georgia 30602, United States ∇ Department of Chemistry, University of Basel, Klingelbergstrasse 80, CH-4056 Basel, Switzerland 7.3. Arylsulfonyl Group 160 7.4. Ketones: 1,5- and 1,6-Hydrogen Abstraction 160 7.5. Carbanion-Mediated Groups 160 7.6. Sisyl and Other Silicon-Based Groups 161 7.7. 2-Hydroxycinnamyl Groups 161 7.8. α-Keto Amides, α,β-Unsaturated Anilides, CONTENTS and Methyl(phenyl)thiocarbamic Acid 162 7.9. Thiochromone S,S-Dioxide 162 1. Introduction 119 7.10. 2-Pyrrolidino-1,4-Benzoquinone Group 162 2. Arylcarbonylmethyl Groups 121 7.11. Triazine and Arylmethyleneimino Groups 162 2.1. Phenacyl and Other Related Arylcarbonyl- 7.12. Xanthene and Pyronin Groups 163 methyl Groups 121 o 7.13. Retro-Cycloaddition Reactions 163 2.2. -Alkylphenacyl Groups 123 8. Sensitized Release 163 2.3. p-Hydroxyphenacyl Groups 125 p 8.1. -

Screening Values for Non-Carcinogenic Hanford Waste Tank Vapor Chemicals That Lack Established Occupational Exposure Limits
PNNL-15640 Screening Values for Non-Carcinogenic Hanford Waste Tank Vapor Chemicals that Lack Established Occupational Exposure Limits TS Poet TJ Mast JL Huckaby February 2006 Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor Battelle Memorial Institute, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or Battelle Memorial Institute. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. PACIFIC NORTHWEST NATIONAL LABORATORY operated by BATTELLE for the UNITED STATES DEPARTMENT OF ENERGY under Contract DE-AC05-76RL01830 Printed in the United States of America Available to DOE and DOE contractors from the Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062; ph: (865) 576-8401 fax: (865) 576-5728 email: [email protected] Available to the public from the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Rd., Springfield, VA 22161 ph: (800) 553-6847 fax: (703) 605-6900 email: [email protected] online ordering: http://www.ntis.gov/ordering.htm This document was printed on recycled paper. -

Aldehydes, Ketones and Carboxylic Acids
1212Unit Objectives AldehydesAldehydesAldehydesAldehydes,,,,,, KKKKKKeeeeeetonestonestonestonestonestones After studying this Unit, you will be able to andandandandandand CarboxylicCarboxylicCarboxylicCarboxylicCarboxylicCarboxylic • write the common and IUPAC names of aldehydes, ketones and carboxylic acids; AAAAAAcidscidscidscidscidscids • write the structures of the compounds containing functional groups namely carbonyl and carboxyl groups; Carbonyl compounds are of utmost importance to organic chemistry. They are constituents of fabrics, flavourings, plastics • describe the important methods and drugs. of preparation and reactions of these classes of compounds; In the previous Unit, you have studied organic • correlate physical properties and compounds with functional groups containing carbon- chemical reactions of aldehydes, oxygen single bond. In this Unit, we will study about the ketones and carboxylic acids, organic compounds containing carbon-oxygen double with their structures; bond (>C=O) called carbonyl group, which is one of the • explain the mechanism of a few most important functional groups in organic chemistry. selected reactions of aldehydes and ketones; In aldehydes, the carbonyl group is bonded to a carbon and hydrogen while in the ketones, it is bonded • understand various factors to two carbon atoms. The carbonyl compounds in which affecting the acidity of carboxylic carbon of carbonyl group is bonded to carbon or acids and their reactions; hydrogen and oxygen of hydroxyl moiety (-OH) are • describe the uses of aldehydes, known as carboxylic acids, while in compounds where ketones and carboxylic acids. carbon is attached to carbon or hydrogen and nitrogen of -NH2 moiety or to halogens are called amides and acyl halides respectively. Esters and anhydrides are derivatives of carboxylic acids. The general formulas of these classes of compounds are given below: 2021–22 Aldehydes, ketones and carboxylic acids are widespread in plants and animal kingdom. -

ABSTRACT JANG, YUJIN. Synthesis of 1-Sulfonylcyclopropanols and Their Application in New Chemical Transformations As Powerful Cy
ABSTRACT JANG, YUJIN. Synthesis of 1-Sulfonylcyclopropanols and Their Application in New Chemical Transformations as Powerful Cyclopropanone Equivalents. (Under the direction of Dr. Vincent Lindsay). Cyclopropanone, a notoriously strained cycloalkanone (Baeyer strain ca. 49 kcal/mol), is a promising three carbon building block in organic synthesis where all three C–C bonds are labile, and can be readily involved in ring-opening or ring-expansion. Despite its tremendous potential as a strained reagent, the difficulties associated with its handling and storage have precluded its widespread use in organic synthesis. For these reasons, a majority of cyclopropanone chemistry in the literature involves the use of equivalents such as hemiketals that can equilibrate to the parent ketone in situ via base-induced α-elimination. Although the classical hemiketals are suitable in some reactions, a number of potential transformations are still inaccessible, as harsh conditions are often required to generate cyclopropanone due to the poor leaving group ability of alkoxides and the immense strain generated in the process. In order to elevate the chemistry of cyclopropanones as common building blocks in synthesis, well-behaved cyclopropanone precursors are highly demanded. Here, we describe the efficient and convenient synthesis and application of unsubstituted 1-sulfonylcyclopropanols as powerful cyclopropanone equivalents. By tuning the electronic and steric nature of leaving group, a variety 1-sulfonylcyclopropanols were furnished, and the relative propensity of their equilibration to cyclopropanone was investigated in a novel pyrazole substitution reaction. With this well-behaved class of surrogates, we explored the C–C bond activation of this strained cycloalkanone in the presence of transition metals and organocatalysts. -

Carbonyl Condensation Reactions
24 Carbonyl Condensation Reactions 24.1 The aldol reaction 24.2 Crossed aldol reactions 24.3 Directed aldol reactions 24.4 Intramolecular aldol reactions 24.5 The Claisen reaction 24.6 The crossed Claisen and related reactions 24.7 The Dieckmann reaction 24.8 The Michael reaction 24.9 The Robinson annulation Ibuprofen is the generic name for the pain reliever known by the trade names of Motrin and Advil. Like aspirin, ibuprofen acts as an anti-infl ammatory agent by blocking the synthesis of prostaglandins from arachidonic acid. One step in a commercial synthesis of ibuprofen involves the reaction of a nucleophilic enolate with an electrophilic carbonyl group. In Chap- ter 24, we learn about the carbon–carbon bond-forming reactions of enolates with carbonyl electrophiles. 916 smi75625_ch24_916-948.indd 916 11/12/09 12:12:52 PM 24.1 The Aldol Reaction 917 In Chapter 24, we examine carbonyl condensations—that is, reactions between two car- bonyl compounds—a second type of reaction that occurs at the α carbon of a carbonyl group. Much of what is presented in Chapter 24 applies principles you have already learned. Many of the reactions may look more complicated than those in previous chapters, but they are fundamen- tally the same. Nucleophiles attack electrophilic carbonyl groups to form the products of nucleo- philic addition or substitution, depending on the structure of the carbonyl starting material. Every reaction in Chapter 24 forms a new carbon–carbon bond at the ` carbon to a carbonyl group, so these reactions are extremely useful in the synthesis of complex natural products. -

Nucleophilic Addition to the Carbonyl Group 6
Nucleophilic addition to the carbonyl group 6 Connections Building on Arriving at Looking forward to • Functional groups, especially the C=O • How and why the C=O group reacts • Additions of organometallic group ch2 with nucleophiles reagents ch9 • Identifying the functional groups in a • Explaining the reactivity of the C=O • Substitution reactions of the C=O molecule spectroscopically ch3 group using molecular orbitals and curly group’s oxygen atom ch11 • How molecular orbitals explain arrows • How the C=O group in derivatives of molecular shapes and functional • What sorts of molecules can be made by carboxylic acids promotes substitution groups ch4 reactions of C=O groups reactions ch10 • How, and why, molecules react together • How acid or base catalysts improve the • C=O groups with an adjacent double and using curly arrows to describe reactivity of the C=O group bond ch22 reactions ch5 Molecular orbitals explain the reactivity of the carbonyl group We are now going to leave to one side most of the reactions you met in the last chapter—we will come back to them all again later in the book. In this chapter we are going to concentrate on just one of them—probably the simplest of all organic reactions—the addition of a nucleo- phile to a carbonyl group. The carbonyl group, as found in aldehydes, ketones, and many other compounds, is without doubt the most important functional group in organic chemis- try, and that is another reason why we have chosen it as our fi rst topic for more detailed study. You met nucleophilic addition to a carbonyl group on pp. -

Photochemical Reactions of Cyclohexanone: Mechanisms and Dynamics Published As Part of the Journal of Physical Chemistry a Virtual Special Issue “Mark S
Article pubs.acs.org/JPCA Photochemical Reactions of Cyclohexanone: Mechanisms and Dynamics Published as part of The Journal of Physical Chemistry A virtual special issue “Mark S. Gordon Festschrift”. † ‡ † ‡ Dorit Shemesh, Sergey A. Nizkorodov, and R. Benny Gerber*, , † Institute of Chemistry and The Fritz Haber Research Center, The Hebrew University, Jerusalem 91904, Israel ‡ Department of Chemistry, University of California, Irvine, California 92697, United States *S Supporting Information ABSTRACT: Photochemistry of carbonyl compounds is of major importance in atmospheric and organic chemistry. The photochemistry of cyclohexanone is studied here using on-the- fly molecular dynamics simulations on a semiempirical multi- reference configuration interaction potential-energy surface to predict the distribution of photoproducts and time scales for their formation. Rich photochemistry is predicted to occur on a picosecond time scale following the photoexcitation of cyclo- hexanone to the first singlet excited state. The main findings include: (1) Reaction channels found experimentally are confirmed by the theoretical simulations, and a new reaction channel is predicted. (2) The majority (87%) of the reactive trajectories start with a ring opening via C−Cα bond cleavage, supporting observations of previous studies. (3) Mechanistic details, time scales, and yields are predicted for all reaction channels. These benchmark results shed light on the photochemistry of isolated carbonyl compounds in the atmosphere and can be extended in the future to photochemistry of more complex atmospherically relevant carbonyl compounds in both gaseous and condensed-phase environments. I. INTRODUCTION representative examples are given), acetaldehyde,8 propanal,9 10 11 The photochemistry of carbonyl compounds is an important butanal and its derivatives, heptanal, and other small − 12 topic in organic and atmospheric chemistry.1 3 Atmospheric aldehydes. -

Copyrighted Material
JWST960-SUBIND JWST960-Smith October 25, 2019 9:5 Printer Name: Trim: 254mm × 178mm SUBJECT INDEX The vast use of transition metal catalysts in organic chemistry makes the citation of every individual metal impractical, so there are limited citations of individual metals. Palladium is one exception where individual citations are common, in keeping with the widespread use of that metal. However, in most cases, the term metal catalyst, or catalyst, metal is used as a heading, usually representing transition metals. A-SE2 mechanism 893 and the steering wheel model acceleration of Diels-Alder reactions 487 151–152 reactions, high pressure A1 mechanism, acetal hydrolysis and universal NMR database 1038 487 155 hydrogen-bonding 1038 A1,3-strain 196 Cahn-Ingold-Prelog system hydrophobic effect 1038 A2 mechanism, acetal hydrolysis 149–152 in water 1038 487 determination 152 ionic liquids 1038 ab initio calculations 36 D/L nomenclature 149 micellular effects 1038 and acidity 346 Kishi’s NMR method 155 microwave irradiation 1038 and antiaromaticity 71 sequence rules 149–152 phosphate 1039 and nonclassical carbocations absolute hardness 64, 359, 361 solid state 1038 427 table 361 ultracentrifuge 1038 norbornyl carbocation 436 absorbents, chiral 168 ultrasound 1038 ab initio studies 248 absorption, and conjugation 317 zeolites 1038 1,2-alkyl shifts in alkyne anions differential, and diastereomers acceleration, Petasis reaction 1349 168 1202 and cubyl carbocation 413 differential, and resolution 169 acenaphthylene, reaction with and SN2 408–409 abstraction,