Progress in Synthesis of Monoglycerides for Use in Food and Pharmaceuticals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
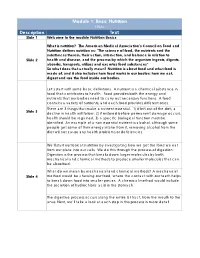
Module 1: Basic Nutrition FINAL Description Text Slide 1 Welcome to the Module Nutrition Basics
Module 1: Basic Nutrition FINAL Description Text Slide 1 Welcome to the module Nutrition Basics What is nutrition? The American Medical Association’s Council on Food and Nutrition defines nutrition as: "The science of food, the nutrients and the substances therein, their action, interaction, and balance in relation to Slide 2 health and disease, and the process by which the organism ingests, digests, absorbs, transports, utilizes and excretes food substances" So what does that actually mean? Nutrition is about food and what food is made of, and it also includes how food works in our bodies: how we eat, digest and use the food inside our bodies. Let’s start with some basic definitions. A nutrient is a chemical substance in food that contributes to health. Food provides both the energy and nutrients that our bodies need to carry out necessary functions. A food contains a variety of nutrients, and each food provides different ones. There are 3 things that make a nutrient essential. 1) if left out of the diet, a Slide 3 decline in health will follow, 2) if restored before permanent damage occurs, health should be regained, 3) a specific biological function must be identified. An example of a non essential nutrient is alcohol, although some people get some of their energy intake from it, removing alcohol from the diet will not cause any health problems or deficiencies. We‘ll start our look at nutrition by investigating how we get the food we eat from our plate into our cells. We do this through the process of digestion. -

Understanding the Basics of Efas
[Nutritional Lipids] Vol. 14 No 9 September 2009 Understanding the Basics of EFAs By Robin Koon, Contributing Editor While knowledge of essential fatty acids (EFAs) is growing among marketers and consumers, reviewing the comprehensive lipid category provides a point of references to delve further into the structure and function of these beneficial fats. Dietary lipids are an essential constituent of the human diet along with carbohydrates, proteins and fiber. Fats are a source of energy, supplying about 9 calories per gram, and make up approximately 40 percent of the body’s normal caloric intake. Lipids are a large and diverse group of naturally occurring organic compounds related by their solubility in non-polar organic solvents (e.g., ether, chloroform, acetone, benzene, etc.) and insolubility in water. In the human diet, triglycerides are the most abundantly consumed form of edible dietary lipids and normally constitute approximately 95 percent of total fat ingested. The remaining 5 percent is in the form of cerebrosides, phospholipids, fatty acids, fatty alcohols, cholesterol, sterols, terpenes, etc. The average human being consumes between 50 and 200 g/d of fat. Triglycerides (TG) are comprised predominantly of fatty acids (present in the form of esters of glycerol), although they are technically a combination of glycerol and three fatty acids, sometimes referred to as triacylglycerol. Glycerol is a three-carbon chain alcohol molecule with chemical formula of C3H803. When combined with one, two or three fatty acids, it forms a monoglyceride, diglyceride or triglyceride, respectively. Triglycerides are generally not well-absorbed in the gut and are enzymatically hydrolyzed, which splits the TG into its components by removing the fatty acids from their glycerol base, so all of these components can be easily absorbed. -

Medium-Chain Fatty Acids and Monoglycerides As Feed Additives for Pig Production: Towards Gut Health Improvement and Feed Pathogen Mitigation Joshua A
Jackman et al. Journal of Animal Science and Biotechnology (2020) 11:44 https://doi.org/10.1186/s40104-020-00446-1 REVIEW Open Access Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation Joshua A. Jackman1*, R. Dean Boyd2,3 and Charles C. Elrod4,5* Abstract Ongoing challenges in the swine industry, such as reduced access to antibiotics and virus outbreaks (e.g., porcine epidemic diarrhea virus, African swine fever virus), have prompted calls for innovative feed additives to support pig production. Medium-chain fatty acids (MCFAs) and monoglycerides have emerged as a potential option due to key molecular features and versatile functions, including inhibitory activity against viral and bacterial pathogens. In this review, we summarize recent studies examining the potential of MCFAs and monoglycerides as feed additives to improve pig gut health and to mitigate feed pathogens. The molecular properties and biological functions of MCFAs and monoglycerides are first introduced along with an overview of intervention needs at different stages of pig production. The latest progress in testing MCFAs and monoglycerides as feed additives in pig diets is then presented, and their effects on a wide range of production issues, such as growth performance, pathogenic infections, and gut health, are covered. The utilization of MCFAs and monoglycerides together with other feed additives such as organic acids and probiotics is also described, along with advances in molecular encapsulation and delivery strategies. Finally, we discuss how MCFAs and monoglycerides demonstrate potential for feed pathogen mitigation to curb disease transmission. Looking forward, we envision that MCFAs and monoglycerides may become an important class of feed additives in pig production for gut health improvement and feed pathogen mitigation. -

Information to Users
INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 00S1079 The survival ofStaphylococcus aureus in abscesses from streptozotocin-induced diabetic mice Harvey, Kevin Michael, Ph.D. -

Deacidification of High-Acid Rice Bran Oil by Reesterification With
Deacidification of High-Acid Rice Bran Oil by Reesterification with Monoglyceride B.K. De and D.K. Bhattacharyya* Department of Chemical Technology, University of Calcutta, Oil Technology Division, Calcutta 700 009, West Bengal, India ABSTRACT: Autocatalytic esterification of free fatty acids (FFA) better process for extracting FFA along with color and peroxide in rice bran oil (RBO) containing high FFA (9.5 to 35.0% w/w) bodies. Bhattacharyya et al. (5) took advantage of this process was examined at a high temperature (210°C) and under low pres- in refining high FFA (20.5 to 56.0%) RBO and showed that a sure (10 mm Hg). The study was conducted to determine the ef- good quality edible-grade RBO could be obtained by this fectiveness of monoglyceride in esterifying the FFA of RBO. The process. study showed that monoglycerides can reduce the FFA level of Chemical or biochemical reesterification of FFA is another degummed, dewaxed, and bleached RBO to an acceptable level approach for the deacidification of high-FFA RBO. Enzymatic (0.5 ± 0.10 to 3.5 ± 0.19% w/w) depending on the FFA content of the crude oil. This allows RBO to be alkali refined, bleached, deacidification (6–10) of vegetable oils by microbial lipases and deodorized or simply deodorized after monoglyceride treat- (i.e., biorefining) has recently received much attention. Lipases ment to obtain a good quality oil. The color of the refined oil is can esterify the FFA to hydroxyl groups containing compounds dependent upon the color of the crude oil used. already present in the oil or to the hydroxyl groups of added Paper no. -

Lymphatic Absorption of Docosahexaenoic Acid Given As Monoglyceride, Diglyceride, Triglyceride, and Ethyl Ester in Rats
JNutrSci Vitaminol, 48, 30-35, 2002 Lymphatic Absorption of Docosahexaenoic Acid Given as Monoglyceride, Diglyceride, Triglyceride, and Ethyl Ester in Rats Fumiaki BANN01,*,Shinji DOIsAKI2,Nobutoshi SHIMIZU2 and Kenshiro FUJIMOTO1 1 Graduate School of Agricultural Science , Tohoku University, 1-1 Tsutsumidori-Amamiyamachi,Aoba, Sendai 981-8555, Japan 2 Central Research Laboratory , Nippon Suisan Kaisha, Ltd., 559-6 Kitano-machi, Hachioji, Tokyo192-0906, Japan (Received March 9, 2001) Summary Lymphatic absorption of docosahexaenoic acids (DHA) given as monoglyc eride (MG), consisting of 1(or 3)-species (91.4%), 2-species (4.2%) and diglyceride (DG)con sisting of 1,3-species (70.8%), 1(or 3),2-species (28.6%), were investigated in comparison with that of triglyceride (TG) and ethyl ester (EE). Rats were infused with a lipid emulsion containing 200mg of DHA-MG, DG, TG, or EE via a gastric cannula. Lymph was collected through the thoracic lymph duct at 2h intervals for 10h and at a single collection from 10 to 30h. Physiological saline containing glucose was infused (2mL/h) throughout the lymph collection. The overall recovery of DHA at 30h after its infusion was significantly higher in the rank order DHA-MG>DG>TG=EE. Moreover, time-dependent changes in re covery rates from 2 to 10h of DHA given as MG were significantly higher than those of the corresponding DG, TG, and EE. These results indicate that DHA-MG and DG are absorbed and transported more effectively than TG and EE forms under restricted water supply, even if they mainly consist of 1(or 3)-species. -

Long Active Injectable Formulations Containing Triacetin
Europaisches Patentamt J European Patent Office OT) Publication number: 0 413 538 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 90308881.3 © int. CIA A61K 31/71, A61K 9/00, A61K 47/14 © Date of filing: 13.08.90 © Priority: 14.08.89 US 393356 © Applicant: MERCK & CO. INC. 126, East Lincoln Avenue P.O. Box 2000 © Date of publication of application: Rahway New Jersey 07065-0900(US) 20.02.91 Bulletin 91/08 © Inventor: Williams, James B. © Designated Contracting States: 4 Coventry Drive CH DE FR GB IT LI NL Freehold, NJ 07728(US) © Representative: Hesketh, Alan, Dr. et al European Patent Department Merck & Co., Inc. Terlings Park Eastwick Road Harlow Essex, CM20 2QR(GB) © Long active injectable formulations containing triacetin. © There is disclosed an injectable formulation containing triacetin and an avermectin compound which has been discovered to provide an unexpectedly long duration of activity. The formulation contains the avermectin active ingredient and at least 50% triacetin and effective levels of the avermectin compounds have been maintained for up to 42 days. Prior injectable formulations provided only 14 days duration of activity. 00 CO If) CO 5 o Q_ LU Xerox Copy Centre EP 0 413 538 A1 BACKGROUND OF THE INVENTION The avermectin series of compounds are potent anthelmintic and antiparasitic agents against internal and external parasites. The natural product avermectins are disclosed in US 4,310,519 to Albers Schonberg et al ., and the 22,23-dihydro avermectin compounds are disclosed in Chabala et al, US 4,199,569. Triacetin (Triacetyl glycerine) is a known formulating agent which is generally recognized as safe by the 5 US Food and Drug Administration. -

Glycerin and the Market
GLYCERIN AND THE MARKET By Valentine Chijioke Mbamalu Approved: Frank Jones Tricia Thomas Professor of Engineering Assistant Professor of Engineering (Chair) (Committee Member) Neslihan Alp William Sutton Professor of Engineering Dean of the College of Engineering and (Committee Member) Computer Science A. Jerald Ainsworth Dean of the Graduate School GLYCERIN AND THE MARKET By Valentine Chijioke Mbamalu A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements of the Degree of Master’s of Engineering The University of Tennessee at Chattanooga Chattanooga, Tennessee May 2013 ii Copyright © 2013 Valentine Chijioke Mbamalu All Rights Reserved iii ABSTRACT Glycerin, a trihydric alcohol, had once enjoyed a good market value but, is now faced with global oversupply and this makes the market volatile. It is a byproduct of biodiesel production thought as an added value to biodiesel operations. It is now faced with an unpredictable market and probably oversupply as an outcome of increased biodiesel production. There are two types of glycerin market; the refined glycerin with its solid price and crude glycerin which is volatile. There are new applications for glycerin being developed or being implemented and it will be a source of strength to the market. This thesis takes an in-depth review of glycerol from its sources to refining and the market. iv ACKNOWLEDGEMENTS First and foremost I thank the Almighty God for the strength and grace He has given me, without which I could not have made it to this point. I express my profound gratitude to my thesis advisor Dr. -

The Properties of Fatty Acids and Monoglycerides. Colin P
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kingston University Research Repository Alternative antimicrobials: the properties of fatty acids and monoglycerides. Colin P. Churchwarda, Raid G. Alanya, and Lori A. S. Snydera* aSchool of Life Sciences, Pharmacy, and Chemistry, Kingston University, Kingston upon Thames, UK *School of Life Sciences, Pharmacy, and Chemistry, Kingston University, Kingston upon Thames, Surrey KT1 2EE, UK; [email protected] Alternative antimicrobials: the properties of fatty acids and monoglycerides. With the rising antibiotic resistance of many bacterial species, alternative treatments are necessary to combat infectious diseases. The World Health Organisation and the U.S. Centers for Disease Control and Prevention have warned that some infections, such as those from Neisseria gonorrhoeae, may be untreatable within a few years. One avenue of exploration is the use of antimicrobial fatty acids and their derivatives for therapeutic prevention or treatment of bacterial infections. Several studies have explored the activity of fatty acids and their derivatives, including monoglycerides against a variety of bacterial species. These are reviewed here, assessing the antimicrobial properties that have been demonstrated and the feasibility of therapeutic applications. Keywords: antibacterial; antiviral; resistance; antimicrobials; infection. Introduction Antibiotics have saved countless lives since they were introduced, yet the spread of bacterial resistances to antibiotics necessitates investigations into other prophylaxis and treatment options. Fatty acids and there derivatives may be alternative antimicrobials worthy of investigation for the post-antibiotic era. The antimicrobial activity of fatty acids and monoglycerides has been known for over 100 years (Lamar 1911), yet the urgent need to identify alternatives in the face of growing antibiotic resistance is driving increased research in this field. -

Propane-1,2-Diyl Diacetate Using Immobilized Lipase B from Candida Antarctica and Pyridinium Chlorochromate As an Oxidizing Agent
International Journal of Molecular Sciences Article Chemoenzymatic Synthesis of the New 3-((2,3-Diacetoxypropanoyl)oxy)propane-1,2-diyl Diacetate Using Immobilized Lipase B from Candida antarctica and Pyridinium Chlorochromate as an Oxidizing Agent Esteban Plata 1,Mónica Ruiz 1, Jennifer Ruiz 1, Claudia Ortiz 2, John J. Castillo 1,* and Roberto Fernández-Lafuente 3,* 1 Escuela de Química, Grupo de investigación en Bioquímica y Microbiología (GIBIM), Edificio Camilo Torres 210, Universidad Industrial de Santander, CEP, 680001 Bucaramanga, Colombia; [email protected] (E.P.); [email protected] (M.R.); [email protected] (J.R.) 2 Escuela de Microbiología, Universidad Industrial de Santander, 680001 Bucaramanga, Colombia; [email protected] 3 ICP-CSIC, Campus UAM-CSIC, Cantoblanco, 28049 Madrid, Spain * Correspondence: [email protected] (J.J.C.); rfl@icp.csic.es (R.F.-L.); Tel.:+57-320-902-6464 (J.J.C.); +34915854804 (R.F.-L.) Received: 3 August 2020; Accepted: 4 September 2020; Published: 5 September 2020 Abstract: To exploit the hydrolytic activity and high selectivity of immobilized lipase B from Candida antarctica on octyl agarose (CALB-OC) in the hydrolysis of triacetin and also to produce new value-added compounds from glycerol, this work describes a chemoenzymatic methodology for the synthesis of the new dimeric glycerol ester 3-((2,3-diacetoxypropanoyl)oxy)propane-1,2-diyl diacetate. According to this approach, triacetin was regioselectively hydrolyzed to 1,2-diacetin with CALB-OC. The diglyceride product was subsequently oxidized with pyridinium chlorochromate (PCC) and a dimeric ester was isolated as the only product. It was found that the medium acidity during the PCC treatment and a high 1,2-diacetin concentration favored the formation of the ester. -

Biofuel That Keeps Glycerol As Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on Alpo4 Support
Energies 2013, 6, 3879-3900; doi:10.3390/en6083879 OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies Article Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support Carlos Luna 1, Enrique Sancho 2, Diego Luna 1,*, Verónica Caballero 1, Juan Calero 1, Alejandro Posadillo 3, Cristóbal Verdugo 4, Felipa M. Bautista 1 and Antonio A. Romero 1 1 Department of Organic Chemistry, University of Cordoba, Campus de Rabanales, Ed. Marie Curie, 14014 Córdoba, Spain; E-Mails: [email protected] (C.L.); [email protected] (V.C.); [email protected] (J.C.); [email protected] (F.M.B.); [email protected] (A.A.R.) 2 Department of Microbiology, University of Córdoba, Campus de Rabanales, Ed. Severo Ochoa, 14014 Córdoba, Spain; E-Mail: [email protected] 3 Seneca Green Catalyst S.L., Campus de Rabanales, 14014 Córdoba, Spain; E-Mail: [email protected] 4 Crystallographic Studies Laboratory, Andalusian Institute of Earth Sciences, CSIC, Avda. Las Palmeras nº4, 18100 Armilla, Granada, Spain; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +34-95-7212065; Fax: +34-95-7212066. Received: 2 July 2013; in revised form: 19 July 2013/ Accepted: 23 July 2013/ Published: 30 July 2013 Abstract: By using pig pancreatic lipase (EC 3.1.1.3 or PPL) as a biocatalyst, covalently immobilized on amorphous AlPO4 support, a new second generation biodiesel was obtained in the transesterification reaction of sunflower oil with ethanol. The resulting biofuel is composed of fatty acid ethyl esters and monoglycerides (FAEE/MG) blended in a 2:1 molar ratio. -

Review of the Antiviral Activity and Pharmacology of Monoglycerides and Implications for Treatment of COVID-19
Review of the antiviral activity and pharmacology of monoglycerides and implications for treatment of COVID-19 Mira Aldridge1 1 correspondence: [email protected] Abstract: Monoglycerides are a class of lipids derived from fatty acids with well established antiviral properties. Monoglycerides are widely used in food processing, cosmetics and in nutritional supplements. This review examines the biochemical and pharmacological properties of monoglycerides and fatty acids as they pertain to COVID-19 pathogenesis. Information on monoglycerides, fatty acids and COVID-19 is presented with sources gathered through PubMed, Elsevier, JSTOR, Oxford University Press Medicine Archive, Wiley, MedRxiv and Google. The antiviral activity of monoglycerides has been documented since the 1970's. In vitro studies have shown that viral concentrations of enveloped viruses were reduced by over 10,000 fold in the presence of monoglycerides such as glycerol monolaurate. Moreover, glycerol monolaurate is widely available and generally recognized as safe (GRAS) by the FDA. The aim of this review is to present an evidence based case for further investigation into this class of compounds for the potential treatment of COVID-19. Keywords: glycerol monolaurate, monolaurin, fatty acids, monoglycerides, lipids, enveloped virus, antiviral, antibacterial, antimicrobial, pathogenesis, COVID-19, ARDS, T-Cells, immune, cytokine, amphiphilic, IL-6 1. Introduction The COVID-19 pandemic is a fast evolving public health and economic emergency. Currently there is no approved vaccine and no preventative medical interventions in use to slow the spread of the disease. Unfortunately long vaccine development timelines preclude their usefulness in alleviating the initial consequences of pandemics. Existing pharmaceutical antiviral or reappropriated drugs may prove effective in reducing severity and length of infection, but substantiative evidence is lacking at this time.