Glycerin and the Market
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
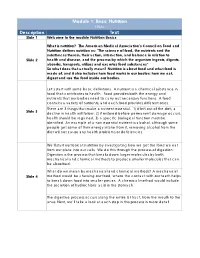
Module 1: Basic Nutrition FINAL Description Text Slide 1 Welcome to the Module Nutrition Basics
Module 1: Basic Nutrition FINAL Description Text Slide 1 Welcome to the module Nutrition Basics What is nutrition? The American Medical Association’s Council on Food and Nutrition defines nutrition as: "The science of food, the nutrients and the substances therein, their action, interaction, and balance in relation to Slide 2 health and disease, and the process by which the organism ingests, digests, absorbs, transports, utilizes and excretes food substances" So what does that actually mean? Nutrition is about food and what food is made of, and it also includes how food works in our bodies: how we eat, digest and use the food inside our bodies. Let’s start with some basic definitions. A nutrient is a chemical substance in food that contributes to health. Food provides both the energy and nutrients that our bodies need to carry out necessary functions. A food contains a variety of nutrients, and each food provides different ones. There are 3 things that make a nutrient essential. 1) if left out of the diet, a Slide 3 decline in health will follow, 2) if restored before permanent damage occurs, health should be regained, 3) a specific biological function must be identified. An example of a non essential nutrient is alcohol, although some people get some of their energy intake from it, removing alcohol from the diet will not cause any health problems or deficiencies. We‘ll start our look at nutrition by investigating how we get the food we eat from our plate into our cells. We do this through the process of digestion. -

Esterifikasi Gliserol Dari Produk Samping Biodiesel Menjadi Triasetin Menggunakan Katalis Zeolit Alam
Esterifikasi Gliserol Dari Produk Samping Biodiesel Menjadi Triasetin Menggunakan Katalis Zeolit Alam Nirmala Sari 1, Zuchra Helwani 2, dan Hari Rionaldo3 Laboratorium Teknologi Oleokimia Program Studi Teknik Kimia S1, Fakultas Teknik Universitas Riau Kampus Binawidya Km. 12,5 Simpang Baru Panam, Pekanbaru 28293 *Email : [email protected] ABSTRACT Glycerol is a by-product of biodiesel production from transesterification reaction generated 10% volume product. The increase of biodiesel production is followed by the increase of the glycerol as by product. Glycerol when esterified with acetic acid formed Triacetin. Triacetin has many uses for food, non-food and additives in biofuel feedstock that is renewable and environmentally friendly. In this study will be make Triacetin from reaction esterification of crude glycerol purified with acetic acid glacial and using natural zeolite catalyst has been activated. Making triacetin performed with a three-neck flask equipped with a condenser, heating mantle, thermometer and magnetic stirred at 100 ° C, 100 mesh size catalyst and reaction time for 4 hours. Process of qualitative analysis using FT-IR instrument has detected the exixtence of Triacetin product. The variables are varied ratio reactant of glycerol and acetic acid, and the concentration catalyst. The highest conversion obtained for 90.02% in reactan ratio mol glycerol and acetic acid 1: 7, catalyst concentration of 3% to weight of acetic acid. Comparison of reagents give real effect to the conversion of glycerol into Triacetin, while the catalyst concentration does not give a significant effect on glycerol conversion be Triacetin. Keywords: acetic acid, esterification, glycerol, Triacetin 1. Pendahuluan Gliserol merupakan produk samping gliserol ester maleat resin. -

Understanding the Basics of Efas
[Nutritional Lipids] Vol. 14 No 9 September 2009 Understanding the Basics of EFAs By Robin Koon, Contributing Editor While knowledge of essential fatty acids (EFAs) is growing among marketers and consumers, reviewing the comprehensive lipid category provides a point of references to delve further into the structure and function of these beneficial fats. Dietary lipids are an essential constituent of the human diet along with carbohydrates, proteins and fiber. Fats are a source of energy, supplying about 9 calories per gram, and make up approximately 40 percent of the body’s normal caloric intake. Lipids are a large and diverse group of naturally occurring organic compounds related by their solubility in non-polar organic solvents (e.g., ether, chloroform, acetone, benzene, etc.) and insolubility in water. In the human diet, triglycerides are the most abundantly consumed form of edible dietary lipids and normally constitute approximately 95 percent of total fat ingested. The remaining 5 percent is in the form of cerebrosides, phospholipids, fatty acids, fatty alcohols, cholesterol, sterols, terpenes, etc. The average human being consumes between 50 and 200 g/d of fat. Triglycerides (TG) are comprised predominantly of fatty acids (present in the form of esters of glycerol), although they are technically a combination of glycerol and three fatty acids, sometimes referred to as triacylglycerol. Glycerol is a three-carbon chain alcohol molecule with chemical formula of C3H803. When combined with one, two or three fatty acids, it forms a monoglyceride, diglyceride or triglyceride, respectively. Triglycerides are generally not well-absorbed in the gut and are enzymatically hydrolyzed, which splits the TG into its components by removing the fatty acids from their glycerol base, so all of these components can be easily absorbed. -

Triglyceride Transesterification in Heterogeneous Reaction System with Calcium Oxide As Catalyst
Rev. Fac. Ing. Univ. Antioquia N.° 57 pp. 7-13. Enero, 2011 Triglyceride transesterification in heterogeneous reaction system with calcium oxide as catalyst Transesterificación de triglicéridos en el sistema de reacción heterogénea con óxido de calcio como catalizador Mónica Becerra Ortega, Aristóbulo Centeno Hurtado, Sonia Azucena Giraldo Duarte* Centro de Investigaciones en Catálisis (CICAT). Escuela de Ingeniería Química. Universidad Industrial de Santander (UIS). Carrera 27 Calle 9. Bucaramanga. Colombia (Recibido el 03 de febrero de 2010. Aceptado el 15 de octubre de 2010) Abstract In this work, the behavior of the CaO as a potential catalyst for the transesterification of triglyceride towards biodiesel production was studied. The effect of the alcohol type, the ratio of alcohol/triacetin, the amount of catalyst, and the chain length of triglyceride on the catalytic behavior of CaO was analyzed. Total conversion was obtained at room temperature with a 6:1 molar ratio of methanol to triacetin over 1% of CaO, after 1 h. It was demonstrated that the whole reaction occurs in heterogeneous phase. During five reaction cycles the CaO maintained a high catalytic activity, showing its good stability. Additionally, it was established that the length of the triglyceride used influenced the transesterification reaction yield due to the steric hindrances and diffusional limitations in the fluid phase. ----- Keywords: Biodiesel, triacetin, basic catalysis, triolein Resumen En este trabajo se estudió el comportamiento del CaO como potencial catalizador en la transesterificación de trigliceridos para la producción de biodiesel. Se analizó el efecto del tipo de alcohol, la relación molar alcohol/triacetina, la cantidad de catalizador y el tamaño de la cadena del triglicérido sobre su comportamiento catalítico. -

Medium-Chain Fatty Acids and Monoglycerides As Feed Additives for Pig Production: Towards Gut Health Improvement and Feed Pathogen Mitigation Joshua A
Jackman et al. Journal of Animal Science and Biotechnology (2020) 11:44 https://doi.org/10.1186/s40104-020-00446-1 REVIEW Open Access Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation Joshua A. Jackman1*, R. Dean Boyd2,3 and Charles C. Elrod4,5* Abstract Ongoing challenges in the swine industry, such as reduced access to antibiotics and virus outbreaks (e.g., porcine epidemic diarrhea virus, African swine fever virus), have prompted calls for innovative feed additives to support pig production. Medium-chain fatty acids (MCFAs) and monoglycerides have emerged as a potential option due to key molecular features and versatile functions, including inhibitory activity against viral and bacterial pathogens. In this review, we summarize recent studies examining the potential of MCFAs and monoglycerides as feed additives to improve pig gut health and to mitigate feed pathogens. The molecular properties and biological functions of MCFAs and monoglycerides are first introduced along with an overview of intervention needs at different stages of pig production. The latest progress in testing MCFAs and monoglycerides as feed additives in pig diets is then presented, and their effects on a wide range of production issues, such as growth performance, pathogenic infections, and gut health, are covered. The utilization of MCFAs and monoglycerides together with other feed additives such as organic acids and probiotics is also described, along with advances in molecular encapsulation and delivery strategies. Finally, we discuss how MCFAs and monoglycerides demonstrate potential for feed pathogen mitigation to curb disease transmission. Looking forward, we envision that MCFAs and monoglycerides may become an important class of feed additives in pig production for gut health improvement and feed pathogen mitigation. -

Information to Users
INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 00S1079 The survival ofStaphylococcus aureus in abscesses from streptozotocin-induced diabetic mice Harvey, Kevin Michael, Ph.D. -

2013 Annual Meeting Abstracts Biotechnology
2013 Annual Meeting Abstracts Biotechnology MONDAY AFTERNOON BIO 1.1/PHO 1: Polar Lipids: Chemistry, Technology, and Applications Chair(s): X. Xu, Wilmar Global R&D Center, China; Aarhus University, Denmark; M. Ahmad, Jina Pharmaceuticals Inc., USA Enzymatic "green" Preparation of Sugar-fatty Acid Esters D. Hayes(1) (1)University of Tennessee, United States of America Saccharide-fatty acid esters are an emerging category of biobased surfactants prepared entirely from renewable resources that are used as emulsifiers in foods, cosmetics, and pharmaceuticals, and possess anticancer and insecticidal properties. Typically, the esters are prepared chemically under harsh condition: temperatures near 200 C, employment of solvents, etc., which can cause undesirable side-reactions and produce waste products. Our group has been investigating the use of lipases and novel bioreactor system design to prepare sugar esters under solvent-free conditions and relatively low temperature: ~65 C. Using a stoichiometric feed of saccharide and fatty acid, our approach achieves 90-95% pure ester on a 10-30 gram scale, which, due to the absence of excess reactants and solvent, will require little or no further downstream purification to achieve industrial specifications. The presentation will provide an overview of our recent work, including an evaluation of its physical properties. Deep Eutectic Solvents: new Opportunities for Lipase-catalyzed Reactions E. DURAND(1), J. LECOMTE(2), B. BAREA(3), P. VILLENEUVE(4) (1)CIRAD, UMR IATE, France (2)CIRAD, UMR IATE, France (3)CIRAD, UMR IATE, France (4)CIRAD, UMR IATE, France In recent years, researchers focused on finding green alternative media to organic solvents for enzyme-catalyzed reactions. -

Deacidification of High-Acid Rice Bran Oil by Reesterification With
Deacidification of High-Acid Rice Bran Oil by Reesterification with Monoglyceride B.K. De and D.K. Bhattacharyya* Department of Chemical Technology, University of Calcutta, Oil Technology Division, Calcutta 700 009, West Bengal, India ABSTRACT: Autocatalytic esterification of free fatty acids (FFA) better process for extracting FFA along with color and peroxide in rice bran oil (RBO) containing high FFA (9.5 to 35.0% w/w) bodies. Bhattacharyya et al. (5) took advantage of this process was examined at a high temperature (210°C) and under low pres- in refining high FFA (20.5 to 56.0%) RBO and showed that a sure (10 mm Hg). The study was conducted to determine the ef- good quality edible-grade RBO could be obtained by this fectiveness of monoglyceride in esterifying the FFA of RBO. The process. study showed that monoglycerides can reduce the FFA level of Chemical or biochemical reesterification of FFA is another degummed, dewaxed, and bleached RBO to an acceptable level approach for the deacidification of high-FFA RBO. Enzymatic (0.5 ± 0.10 to 3.5 ± 0.19% w/w) depending on the FFA content of the crude oil. This allows RBO to be alkali refined, bleached, deacidification (6–10) of vegetable oils by microbial lipases and deodorized or simply deodorized after monoglyceride treat- (i.e., biorefining) has recently received much attention. Lipases ment to obtain a good quality oil. The color of the refined oil is can esterify the FFA to hydroxyl groups containing compounds dependent upon the color of the crude oil used. already present in the oil or to the hydroxyl groups of added Paper no. -

Chemical Kinetics for Synthesis of Triacetin from Biodiesel Byproduct
www.ccsenet.org/ijc International Journal of Chemistry Vol. 4, No. 2; April 2012 Chemical Kinetics for Synthesis of Triacetin from Biodiesel Byproduct Zahrul Mufrodi Department of Chemical Engineering, Ahmad Dahlan University 9 Kapas Street, Yogyakarta 55166, Indonesia Tel: 62-274-743-6596 E-mail: [email protected] Sutijan, Rochmadi & Arief Budiman Department of Chemical Engineering, Gadjah Mada University 2 Grafika Street, Yogyakarta 55281, Indonesia Received: December 9, 2011 Accepted: January 29, 2012 Published: April 1, 2012 doi:10.5539/ijc.v4n2p101 URL: http://dx.doi.org/10.5539/ijc.v4n2p101 The research is financed by KKP3T department of agriculture and Department of national education Indonesia Abstract The reaction kinetic of the glycerol acetylation with acetic acid catalyzed by sulfuric acid has been studied in the frame of continuous triacetin production. Glycerol, acetic acid and sulfuric acid catalyst were reacted in a batch reactor, in order to get reaction kinetics data. The mole ratio of catalyst to glycerol and temperature were studied during the experience. This study concluded that the selectivity of triacetin increased with increase in mole ratio of catalyst to glycerol. Increasing temperatures lead to increase selectivity of triacetin. It will decreased at the time of acetic acid has begun to evaporate. Triacetin synthesis is an exothermic reaction, a higher reaction temperature will cause in shifting the balance toward formation of reactants. This needs to be anticipated by taking one of the products so that the equilibrium shifting toward product formation. Keywords: Reaction kinetic, Glycerol, Acetylation, Triacetin, Acetic acid 1. Introduction Needs of oil energy sources from fossil fuels are increasing, while inventories are running low. -

PARTIAL CHARACTERIZATION of LIPASE from COCOA BEANS (Theobroma Cacao
448 Indo. J. Chem., 2008, 8 (3), 448 - 453 PARTIAL CHARACTERIZATION OF LIPASE FROM COCOA BEANS (Theobroma cacao. L.) OF CLONE PBC 159 Ratna Agung Samsumaharto Faculty of Biology, Setia Budi University, Jl. Let. Jend. Sutoyo Mojosongo – Surakarta 57127 Received 3 April 2008; Accepted 15 October 2008 ABSTRACT A study was carried out to characterize the cocoa lipase from cocoa beans (Theobroma cacao, L.) of clone PBC 159. The optimum temperature of cocoa lipase was 30-40 °C and the pH optimum was 7.0-8.0. The moleculer weight of the lipase enzyme was in between 45-66 kDa. The results indicate that Km value for cocoa bean lipase was 2.63 mM, when trimyristin was used as a substrate. The incubation of cocoa bean lipase with triolein and tributyrin (as substrate) yielded Km of 11.24 and 35.71 mM, respectively. The Vmax value obtained from the incubation of the lipase with a wide range of substrates, including tributyrin, trimyristin and triolein, are expressed as µmole acid/min/mg protein for cocoa lipase. Vmax values decreased with the increase in the triacylglycerol chain-length, with Vmax values of 27.78, 13.16 and 11.63 µmole acid/min/mg protein when incubated with tributyrin, trimyristin and triolein, respectively. Inhibition of lipase occurred in the presence of diisopropyl flourophosphate, N- bromosuccinimide and 5,5-dithiobis-(-2-nitrobenzoic acid). Keywords: characterization, lipase, cocoa beans INTRODUCTION According to Hassan [8] the lipase showed that the optimum temperature for oil palm mesocarp lipase Lipases are ester hydrolases or esterases since activity range between 20.0 to 32.5 °C. -

A DFT Investigation of Methanolysis and Hydrolysis of Triacetin Abstract 1
A DFT investigation of methanolysis and hydrolysis of triacetin Taweetham Limpanuparba, Kraiwan Punyainb, Yuthana Tantirungrotechaic,d,* aDepartment of Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand bDepartment of Chemistry, Faculty of Science, Naresuan University, Phitsanulok 65000, Thailand cNational Nanotechnology Center (NANOTEC), National Science and Technology Development Agency, Pathumthani 12120, Thailand dTheoretical Chemistry Laboratory, Faculty of Science, Mahidol University, Salaya, Nakhon Pathom 73170, Thailand * Corresponding author at: Address: National Nanotechnology Center (NANOTEC), National Science and Technology Development Agency, Pathumthani 12120, Thailand. This paper was subsequently accepted for publication in Journal of Molecular Structure: THEOCHEM Volume 955, Issues 1–3, 15 September 2010, Pages 23–32 Refer to http://dx.doi.org/10.1016/j.theochem.2010.05.022 for the final published version. Abstract The thermodynamic and kinetic aspects of the methanolysis and hydrolysis reactions of glycerol triacetate or triacetin, a model triacylglycerol compound, were investigated by using Density Functional Theory (DFT) at the B3LYP/6-31++G(d,p) level of calculation. Twelve elementary steps of triacetin methanolysis were studied under acid-catalyzed and base-catalyzed conditions. The mechanism of acid-catalyzed methanolysis reaction which has not been reported yet for any esters was proposed. The effects of substitution, methanolysis/hydrolysis position, solvent and face of nucleophilic attack on the free energy of reaction and activation energy were examined. The prediction confirmed the facile position at the middle position of glycerol observed by NMR techniques. The calculated activation energy and the trends of those factors agree with existing experimental observations in biodiesel production. 1. Introduction Triacylglyceride (TG) is a major constituent of naturally available oil and fat. -

Lymphatic Absorption of Docosahexaenoic Acid Given As Monoglyceride, Diglyceride, Triglyceride, and Ethyl Ester in Rats
JNutrSci Vitaminol, 48, 30-35, 2002 Lymphatic Absorption of Docosahexaenoic Acid Given as Monoglyceride, Diglyceride, Triglyceride, and Ethyl Ester in Rats Fumiaki BANN01,*,Shinji DOIsAKI2,Nobutoshi SHIMIZU2 and Kenshiro FUJIMOTO1 1 Graduate School of Agricultural Science , Tohoku University, 1-1 Tsutsumidori-Amamiyamachi,Aoba, Sendai 981-8555, Japan 2 Central Research Laboratory , Nippon Suisan Kaisha, Ltd., 559-6 Kitano-machi, Hachioji, Tokyo192-0906, Japan (Received March 9, 2001) Summary Lymphatic absorption of docosahexaenoic acids (DHA) given as monoglyc eride (MG), consisting of 1(or 3)-species (91.4%), 2-species (4.2%) and diglyceride (DG)con sisting of 1,3-species (70.8%), 1(or 3),2-species (28.6%), were investigated in comparison with that of triglyceride (TG) and ethyl ester (EE). Rats were infused with a lipid emulsion containing 200mg of DHA-MG, DG, TG, or EE via a gastric cannula. Lymph was collected through the thoracic lymph duct at 2h intervals for 10h and at a single collection from 10 to 30h. Physiological saline containing glucose was infused (2mL/h) throughout the lymph collection. The overall recovery of DHA at 30h after its infusion was significantly higher in the rank order DHA-MG>DG>TG=EE. Moreover, time-dependent changes in re covery rates from 2 to 10h of DHA given as MG were significantly higher than those of the corresponding DG, TG, and EE. These results indicate that DHA-MG and DG are absorbed and transported more effectively than TG and EE forms under restricted water supply, even if they mainly consist of 1(or 3)-species.