Lipolysis: Pathway Under Construction Rudolf Zechner, Juliane G
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
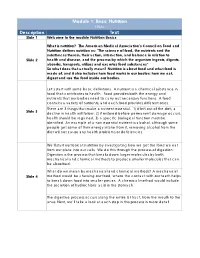
Module 1: Basic Nutrition FINAL Description Text Slide 1 Welcome to the Module Nutrition Basics
Module 1: Basic Nutrition FINAL Description Text Slide 1 Welcome to the module Nutrition Basics What is nutrition? The American Medical Association’s Council on Food and Nutrition defines nutrition as: "The science of food, the nutrients and the substances therein, their action, interaction, and balance in relation to Slide 2 health and disease, and the process by which the organism ingests, digests, absorbs, transports, utilizes and excretes food substances" So what does that actually mean? Nutrition is about food and what food is made of, and it also includes how food works in our bodies: how we eat, digest and use the food inside our bodies. Let’s start with some basic definitions. A nutrient is a chemical substance in food that contributes to health. Food provides both the energy and nutrients that our bodies need to carry out necessary functions. A food contains a variety of nutrients, and each food provides different ones. There are 3 things that make a nutrient essential. 1) if left out of the diet, a Slide 3 decline in health will follow, 2) if restored before permanent damage occurs, health should be regained, 3) a specific biological function must be identified. An example of a non essential nutrient is alcohol, although some people get some of their energy intake from it, removing alcohol from the diet will not cause any health problems or deficiencies. We‘ll start our look at nutrition by investigating how we get the food we eat from our plate into our cells. We do this through the process of digestion. -

Amphibolic Nature of Krebs Cycle
Amphibolic nature of Krebs Cycle How what we are is what we eat • In aerobic organisms, the citric acid cycle is an amphibolic pathway, one that serves in both catabolic and anabolic processes. • Since the citric acid does both synthesis (anabolic) and breakdown (catabolic) activities, it is called an amphibolic pathway • The citric acid cycle is amphibolic (i.e it is both anabolic and catabolic in its function). • It is said to be an AMPHIBOLIC pathway, because it functions in both degradative or catabolic and biosynthetic or anabolic reactions (amphi = both) A central metabolic pathway or amphibolic pathway is a set of reactions which permit the interconversion of several metabolites, and represents the end of the catabolism and the beginning of anabolism • The KREBS CYCLE or citric acid cycle is a series of reactions that degrades acetyl CoA to yield carbon dioxide, and energy, which is used to produce NADH, H+ and FADH. • The KREBS CYCLE connects the catabolic pathways that begin with the digestion and degradation of foods in stages 1 and 2 with the oxidation of substrates in stage 3 that generates most of the energy for ATP synthesis. • The citric acid cycle is the final common pathway in the oxidation of fuel molecules. In stage 3 of metabolism, citric acid is a final common catabolic intermediate in the form of acetylCoA. • This is why the citric acid cycle is called a central metabolic pathway. Anaplerosis and Cataplerosis Anaplerosis is a series of enzymatic reactions in which metabolic intermediates enter the citric acid cycle from the cytosol. Cataplerosis is the opposite, a process where intermediates leave the citric acid cycle and enter the cytosol. -

The Citric Acid Cycle the Catabolism of Acetyl-Coa
Al-Sham Private University Faculty Of Pharmacy The Citric Acid Cycle The Catabolism of Acetyl-CoA Lecturer Prof. Abboud Al-Saleh 1 Prof.Abboud AL-Saleh 10/1/2018 BIOMEDICAL IMPORTANCE • The citric acid cycle (Krebs cycle, tricarboxylic acid cycle) is a series of reactions in mitochondria that oxidize acetyl residues (as acetyl-CoA) and reduce coenzymes that upon reoxidation are linked to the formation of ATP. • TCA is the final common pathway for the aerobic oxidation of carbohydrate, lipid, and protein because glucose, fatty acids, and most amino acids are metabolized to acetyl-CoA or intermediates of the cycle. 2 Prof.Abboud AL-Saleh 10/1/2018 • TCA also has a central role in gluconeogenesis, lipogenesis, and interconversion of amino acids. Many of these processes occur in most tissues, but the liver is the only tissue in which all occur to a significant extent. • The repercussions are therefore profound when, for example, large numbers of hepatic cells are damaged as in acute hepatitis or as in cirrhosis. • Very few, if any, genetic abnormalities of TCA enzymes have been reported; such abnormalities would be incompatible with life or normal development 3 Prof.Abboud AL-Saleh 10/1/2018 Summary • The cycle starts with reaction between the acetyl moiety of acetyl-CoA and the four-carbon dicarboxylic acid oxaloacetate, forming a six-carbon tricarboxylic acid, citrate. • In the subsequent reactions, two molecules of CO2 are released and oxaloacetate is regenerated (Figure). • Only a small quantity of oxaloacetate is needed for the oxidation of a large quantity of acetyl-CoA. • oxaloacetate may be considered to play a catalytic role. -

Catabolism Iii
Nitrogen Catabolism Glycogenolysis Protein Fat catabolism Catabolism Fatty Acid Amino-acid GlycolysisDegradation catabolism Pyruvate Oxidation Krebs' Cycle Phosphorylation Oxidative CATABOLISM III: Digestion and Utilization of Proteins • Protein degradation • Protein turnover – The ubiquitin pathway – Protein turnover is tightly regulated • Elimination of nitrogen – By fish, flesh and fowl – How is the N of amino acids liberated and eliminated? • How are amino acids oxidized for energy 1 Protein Catabolism Sources of AMINO ACIDS: •Dietary amino acids that exceed body’s protein synthesis needs •Excess amino acids from protein turnover (e.g., proteolysis and regeneration of proteins) •Proteins in the body can be broken down (muscle wasting) to supply amino acids for energy when carbohydrates are scarce (starvation, diabetes mellitus). •Carnivores use amino acids for energy more than herbivores, plants, and most microorganisms Protein Catabolism The Digestion Pathway • Pro-enzymes are secreted (zymogens) and the environment activates them by specific proteolysis. • Pepsin hydrolyzes protein into peptides in the stomach. • Trypsin and chymotrypsin hydrolyze proteins and larger peptides into smaller peptides in the small intestine. • Aminopeptidase and carboxypeptidases A and B degrade peptides into amino acids in the small intestine. 2 Protein Catabolism The Lysosomal Pathway • Endocytosis, either receptor-mediated, phagocytosis, or pinocytosis engulfs extra- cellular proteins into vesicles. • These internal vesicles fuse as an early endosome. • This early endosome is acidified by the KFERQ Substrates vATPase (“v” for vesicular). • Components that are recycled, like receptors, HSPA8 are sequestered in smaller vesicles to create Co-chaperones the multivesicular body (MVB), sometimes called a late endosome. • If set for degradation, it will fuse with a KFERQ primary lysosome (red) which contains many cathepsin-type proteases. -

Tepzz¥5Z5 8 a T
(19) TZZ¥Z___T (11) EP 3 505 181 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 03.07.2019 Bulletin 2019/27 A61K 38/46 (2006.01) C12N 9/16 (2006.01) (21) Application number: 18248241.4 (22) Date of filing: 28.12.2018 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • DICKSON, Patricia GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Torrance, CA California 90502 (US) PL PT RO RS SE SI SK SM TR • CHOU, Tsui-Fen Designated Extension States: Torrance, CA California 90502 (US) BA ME • EKINS, Sean Designated Validation States: Brooklyn, NY New York 11215 (US) KH MA MD TN • KAN, Shih-Hsin Torrance, CA California 90502 (US) (30) Priority: 28.12.2017 US 201762611472 P • LE, Steven 05.04.2018 US 201815946505 Torrance, CA California 90502 (US) • MOEN, Derek R. (71) Applicants: Torrance, CA California 90502 (US) • Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (74) Representative: J A Kemp Torrance, CA 90502 (US) 14 South Square • Phoenix Nest Inc. Gray’s Inn Brooklyn NY 11215 (US) London WC1R 5JJ (GB) (54) PREPARATION OF ENZYME REPLACEMENT THERAPY FOR MUCOPOLYSACCHARIDOSIS IIID (57) The present disclosure relates to compositions for use in a method of treating Sanfilippo syndrome (also known as Sanfilippo disease type D, Sanfilippo D, mu- copolysaccharidosis type IIID, MPS IIID). The method can entail injecting to the spinal fluid of a MPS IIID patient an effective amount of a composition comprising a re- combinant human acetylglucosamine-6-sulfatase (GNS) protein comprising the amino acid sequence of SEQ ID NO: 1 or an amino acid sequence having at least 90% sequence identity to SEQ ID NO: 1 and having the en- zymatic activity of the human GNS protein. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing -

Understanding the Basics of Efas
[Nutritional Lipids] Vol. 14 No 9 September 2009 Understanding the Basics of EFAs By Robin Koon, Contributing Editor While knowledge of essential fatty acids (EFAs) is growing among marketers and consumers, reviewing the comprehensive lipid category provides a point of references to delve further into the structure and function of these beneficial fats. Dietary lipids are an essential constituent of the human diet along with carbohydrates, proteins and fiber. Fats are a source of energy, supplying about 9 calories per gram, and make up approximately 40 percent of the body’s normal caloric intake. Lipids are a large and diverse group of naturally occurring organic compounds related by their solubility in non-polar organic solvents (e.g., ether, chloroform, acetone, benzene, etc.) and insolubility in water. In the human diet, triglycerides are the most abundantly consumed form of edible dietary lipids and normally constitute approximately 95 percent of total fat ingested. The remaining 5 percent is in the form of cerebrosides, phospholipids, fatty acids, fatty alcohols, cholesterol, sterols, terpenes, etc. The average human being consumes between 50 and 200 g/d of fat. Triglycerides (TG) are comprised predominantly of fatty acids (present in the form of esters of glycerol), although they are technically a combination of glycerol and three fatty acids, sometimes referred to as triacylglycerol. Glycerol is a three-carbon chain alcohol molecule with chemical formula of C3H803. When combined with one, two or three fatty acids, it forms a monoglyceride, diglyceride or triglyceride, respectively. Triglycerides are generally not well-absorbed in the gut and are enzymatically hydrolyzed, which splits the TG into its components by removing the fatty acids from their glycerol base, so all of these components can be easily absorbed. -

Comparative Study of Idursulfase Beta and Idursulfase in Vitro and in Vivo
Journal of Human Genetics (2017) 62, 167–174 OPEN Official journal of the Japan Society of Human Genetics www.nature.com/jhg ORIGINAL ARTICLE Comparative study of idursulfase beta and idursulfase in vitro and in vivo Chihwa Kim1, Jinwook Seo2, Yokyung Chung2, Hyi-Jeong Ji3, Jaehyeon Lee2, Jongmun Sohn2, Byoungju Lee2 and Eui-cheol Jo1 Hunter syndrome is an X-linked lysosomal storage disease caused by a deficiency in the enzyme iduronate-2-sulfatase (IDS), leading to the accumulation of glycosaminoglycans (GAGs). Two recombinant enzymes, idursulfase and idursulfase beta are currently available for enzyme replacement therapy for Hunter syndrome. These two enzymes exhibited some differences in various clinical parameters in a recent clinical trial. Regarding the similarities and differences of these enzymes, previous research has characterized their biochemical and physicochemical properties. We compared the in vitro and in vivo efficacy of the two enzymes on patient fibroblasts and mouse model. Two enzymes were taken up into the cell and degraded GAGs accumulated in fibroblasts. In vivo studies of two enzymes revealed similar organ distribution and decreased urinary GAGs excretion. Especially, idursulfase beta exhibited enhanced in vitro efficacy for the lower concentration of treatment, in vivo efficacy in the degradation of tissue GAGs and improvement of bones, and revealed lower anti-drug antibody formation. A biochemical analysis showed that both enzymes show largely a similar glycosylation pattern, but the several peaks were different and quantity of aggregates of idursulfase beta was lower. Journal of Human Genetics (2017) 62, 167–174; doi:10.1038/jhg.2016.133; published online 10 November 2016 INTRODUCTION secreted protein and contains eight N-linked glycosylation sites at Mucopolysaccharidosis II (MPS II, Hunter syndrome; OMIM 309900) positions 31, 115, 144, 246, 280, 325, 513 and 537. -

Medium-Chain Fatty Acids and Monoglycerides As Feed Additives for Pig Production: Towards Gut Health Improvement and Feed Pathogen Mitigation Joshua A
Jackman et al. Journal of Animal Science and Biotechnology (2020) 11:44 https://doi.org/10.1186/s40104-020-00446-1 REVIEW Open Access Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation Joshua A. Jackman1*, R. Dean Boyd2,3 and Charles C. Elrod4,5* Abstract Ongoing challenges in the swine industry, such as reduced access to antibiotics and virus outbreaks (e.g., porcine epidemic diarrhea virus, African swine fever virus), have prompted calls for innovative feed additives to support pig production. Medium-chain fatty acids (MCFAs) and monoglycerides have emerged as a potential option due to key molecular features and versatile functions, including inhibitory activity against viral and bacterial pathogens. In this review, we summarize recent studies examining the potential of MCFAs and monoglycerides as feed additives to improve pig gut health and to mitigate feed pathogens. The molecular properties and biological functions of MCFAs and monoglycerides are first introduced along with an overview of intervention needs at different stages of pig production. The latest progress in testing MCFAs and monoglycerides as feed additives in pig diets is then presented, and their effects on a wide range of production issues, such as growth performance, pathogenic infections, and gut health, are covered. The utilization of MCFAs and monoglycerides together with other feed additives such as organic acids and probiotics is also described, along with advances in molecular encapsulation and delivery strategies. Finally, we discuss how MCFAs and monoglycerides demonstrate potential for feed pathogen mitigation to curb disease transmission. Looking forward, we envision that MCFAs and monoglycerides may become an important class of feed additives in pig production for gut health improvement and feed pathogen mitigation. -

Sanfilippo Disease Type D: Deficiency of N-Acetylglucosamine-6- Sulfate Sulfatase Required for Heparan Sulfate Degradation
Proc. Nat!. Acad. Sci. USA Vol. 77, No. 11, pp. 6822-6826, November 1980 Medical Sciences Sanfilippo disease type D: Deficiency of N-acetylglucosamine-6- sulfate sulfatase required for heparan sulfate degradation (mucopolysaccharidosis/keratan sulfate/lysosomes) HANS KRESSE, EDUARD PASCHKE, KURT VON FIGURA, WALTER GILBERG, AND WALBURGA FUCHS Institute of Physiological Chemistry, Waldeyerstrasse 15, D-4400 Mfinster, Federal Republic of Germany Communicated by Elizabeth F. Neufeld, July 10, 1980 ABSTRACT Skin fibroblasts from two patients who had lippo syndromes and excreted excessive amounts of heparan symptoms of the Sanfilippo syndrome (mucopolysaccharidosis sulfate and keratan sulfate in the urine. His fibroblasts were III) accumulated excessive amounts of hean sulfate and were unable to desulfate N-acetylglucosamine-6-sulfate and the unable to release sulfate from N-acety lucosamine)--sulfate linkages in heparan sulfate-derived oligosaccharides. Keratan corresponding sugar alcohol (17, 18). It was therefore suggested sulfate-derived oligosaccharides bearing the same residue at that N-acetylglucosamine-6-sulfate sulfatase is involved in the the nonreducing end and p-nitrophenyl6sulfo-2-acetamido- catabolism of both types of macromolecules. 2-deoxy-D-ucopyranoside were degraded normally. Kinetic In this paper, we describe a new disease, tentatively desig- differences between the sulfatase activities of normal fibro- nated Sanfilippo disease type D, that is characterized by the blasts were found. These observations suggest that N-acetyl- excessive excretion glucosamine4-6sulfate sulfatase activities degrading heparan clinical features of the Sanfilippo syndrome, sulfate and keratan sulfate, respectively, can be distinguished. of heparan sulfate, and the inability to release inorganic sulfate It is the activity directed toward heparan sulfate that is deficient from N-acetylglucosamine-6-sulfate residues of heparan sul- in these patients; we propose that this deficiency causes Sanfi- fate-derived oligosaccharides. -

Letters to Nature
letters to nature Received 7 July; accepted 21 September 1998. 26. Tronrud, D. E. Conjugate-direction minimization: an improved method for the re®nement of macromolecules. Acta Crystallogr. A 48, 912±916 (1992). 1. Dalbey, R. E., Lively, M. O., Bron, S. & van Dijl, J. M. The chemistry and enzymology of the type 1 27. Wolfe, P. B., Wickner, W. & Goodman, J. M. Sequence of the leader peptidase gene of Escherichia coli signal peptidases. Protein Sci. 6, 1129±1138 (1997). and the orientation of leader peptidase in the bacterial envelope. J. Biol. Chem. 258, 12073±12080 2. Kuo, D. W. et al. Escherichia coli leader peptidase: production of an active form lacking a requirement (1983). for detergent and development of peptide substrates. Arch. Biochem. Biophys. 303, 274±280 (1993). 28. Kraulis, P.G. Molscript: a program to produce both detailed and schematic plots of protein structures. 3. Tschantz, W. R. et al. Characterization of a soluble, catalytically active form of Escherichia coli leader J. Appl. Crystallogr. 24, 946±950 (1991). peptidase: requirement of detergent or phospholipid for optimal activity. Biochemistry 34, 3935±3941 29. Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and (1995). the thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281±296 (1991). 4. Allsop, A. E. et al.inAnti-Infectives, Recent Advances in Chemistry and Structure-Activity Relationships 30. Meritt, E. A. & Bacon, D. J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505± (eds Bently, P. H. & O'Hanlon, P. J.) 61±72 (R. Soc. Chem., Cambridge, 1997). -

Information to Users
INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 00S1079 The survival ofStaphylococcus aureus in abscesses from streptozotocin-induced diabetic mice Harvey, Kevin Michael, Ph.D.