Name: Edhelper the Electromagnetic Spectrum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Know the Color Wheel Primary Color
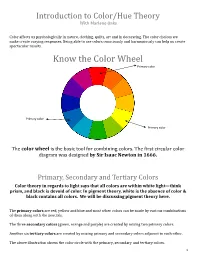
Introduction to Color/Hue Theory With Marlene Oaks Color affects us psychologically in nature, clothing, quilts, art and in decorating. The color choices we make create varying responses. Being able to use colors consciously and harmoniously can help us create spectacular results. Know the Color Wheel Primary color Primary color Primary color The color wheel is the basic tool for combining colors. The first circular color diagram was designed by Sir Isaac Newton in 1666. Primary, Secondary and Tertiary Colors Color theory in regards to light says that all colors are within white light—think prism, and black is devoid of color. In pigment theory, white is the absence of color & black contains all colors. We will be discussing pigment theory here. The primary colors are red, yellow and blue and most other colors can be made by various combinations of them along with the neutrals. The three secondary colors (green, orange and purple) are created by mixing two primary colors. Another six tertiary colors are created by mixing primary and secondary colors adjacent to each other. The above illustration shows the color circle with the primary, secondary and tertiary colors. 1 Warm and cool colors The color circle can be divided into warm and cool colors. Warm colors are energizing and appear to come forward. Cool colors give an impression of calm, and appear to recede. White, black and gray are considered to be neutral. Tints - adding white to a pure hue: Terms about Shades - adding black to a pure hue: hue also known as color Tones - adding gray to a pure hue: Test for color blindness NOTE: Color theory is vast. -

Violet-Green Swallow
Breeding Habitat Use Profile Habitats Used in Arizona Primary: Montane Riparian Secondary: Montane Forests, locally Upper Sonoran Desert Key Habitat Parameters Plant Composition Most montane forest types, often with some element of riparian, wetland, open water or 8 other moist habitat types Plant Density and Unknown Size Violet-green Swallow, photo by ©George Andrejko Microhabitat Snags, live trees, or cliffs for nesting, mesic Features areas with high insect productivity for forag- Conservation Profile ing 8; in wooded landscapes, often noted foraging and nesting near forest clearings Species Concerns and edges. Climate Change (Droughts) Increasing Fire Frequency Landscape Largely unknown, but must include some Timber Harvesting Practices old-growth forests or cliffs Conservation Status Lists Elevation Range in Arizona USFWS 1 No 3,200 – 10,500 feet, locally to 1,200 feet 9 AZGFD 2 No Density Estimate DoD 3 No Territory Size: Unknown BLM 4 No Density: Unknown, sometimes occurs in loose colonies 8 PIF Watch List 5b No PIF Regional Concern 5a No Migratory Bird Treaty Act Natural History Profile Covered Seasonal Distribution in Arizona PIF Breeding Population Size Estimates 6 Breeding April – early August, desert nesting may Arizona 710,000 ◑ begin in March 9 Global 7,200,000 ◑ Migration February – April; August – mid-October 9 9.93% Percent in Arizona Winter Rare, very small numbers 5b PIF Population Goal Nest and Nesting Habits Maintain 8 Type of Nest Cavity or crevice Trends in Arizona Nest Substrate Tree, rock, or cliff; also artificial -

Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC
Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC Introduction Why does the color of a copper chloride solution appear blue? As the white light hits the paint, which colors does the solution absorb and which colors does it transmit? In this activity students will observe the basic principles of absorption spectroscopy based on absorbance and transmittance of visible light. Concepts • Spectroscopy • Visible light spectrum • Absorbance and transmittance • Quantized electron energy levels Background The visible light spectrum (380−750 nm) is the light we are able to see. This spectrum is often referred to as “ROY G BIV” as a mnemonic device for the order of colors it produces. Violet has the shortest wavelength (about 400 nm) and red has the longest wavelength (about 650–700 nm). Many common chemical solutions can be used as filters to demonstrate the principles of absorption and transmittance of visible light in the electromagnetic spectrum. For example, copper(II) chloride (blue), ammonium dichromate (orange), iron(III) chloride (yellow), and potassium permanganate (red) are all different colors because they absorb different wave- lengths of visible light. In this demonstration, students will observe the principles of absorption spectroscopy using a variety of different colored solutions. Food coloring will be substituted for the orange and yellow chemical solutions mentioned above. Rare earth metal solutions, erbium and praseodymium chloride, will be used to illustrate line absorption spectra. Materials Copper(II) chloride solution, 1 M, 85 mL Diffraction grating, holographic, 14 cm × 14 cm Erbium chloride solution, 0.1 M, 50 mL Microchemistry solution bottle, 50 mL, 6 Potassium permanganate solution (KMnO4), 0.001 M, 275 mL Overhead projector and screen Praseodymium chloride solution, 0.1 M, 50 mL Red food dye Water, deionized Stir rod, glass Beaker, 250-mL Tape Black construction paper, 12 × 18, 2 sheets Yellow food dye Colored pencils Safety Precautions Copper(II) chloride solution is toxic by ingestion and inhalation. -

Monochromatic: Red Orange Yellow Green Blue Violet Complementary
Monochromatic: Split Complementary: Red Red, Yellow-Green, Blue-Green Orange Red-Orange, Green, Blue Yellow Orange, Blue-Green, Blue-Violet Green Yellow-Orange, Blue, Violet Blue Yellow, Blue-Violet, Red-Violet Violet Yellow-Green, Violet, Red Green, Red-Violet, Red-Orange Complementary: Blue-Green, Red, Orange Red & Green Blue, Red-Orange, Yellow-Orange Red-Orange & Blue-Green Blue-Violet, Orange, Yellow Orange & Blue Violet, Yellow-Orange, Yellow-Green Yellow-Orange & Blue-Violet Red-Violet, Yellow, Green Yellow & Violet Yellow-Green & Red-Violet Tetradic: Red, Yellow, Green, Violet Triadic: Red, Yellow-Orange, Green, Blue-Violet Red, Yellow, Blue Red-Orange, Blue-Green, Yellow-Orange, Blue- Red-Orange, Yellow-Green, Blue-Violet Violet Orange, Green, Violet Red-Orange, Blue-Green, Yellow, Violet Yellow-Orange, Blue-Green, Red-Violet Orange, Blue, Green, Red Orange, Blue, Yellow-Green, Red-Violet Analogous: Yellow-Orange, Blue-Violet, Green, Red Red, Red Orange, Orange Yellow, Violet, Blue, Orange Red-Orange, Orange, Yellow-Orange Yellow-Green, Red-Violet, Blue-Green, Red- Orange, Yellow-Orange, Yellow Orange Yellow-Orange, Yellow, Yellow-Green Yellow, Yellow-Green, Green Yellow-Green, Green, Blue-Green Green, Blue-Green, Blue Blue-Green, Blue, Blue-Violet Blue, Blue-Violet, Violet Blue-Violet, Violet, Red-Violet Violet, Red-Violet, Red Red-Violet, Red, Red-Orange . -

The Electromagnetic Spectrum
The Electromagnetic Spectrum Wavelength/frequency/energy MAP TAP 2003-2004 The Electromagnetic Spectrum 1 Teacher Page • Content: Physical Science—The Electromagnetic Spectrum • Grade Level: High School • Creator: Dorothy Walk • Curriculum Objectives: SC 1; Intro Phys/Chem IV.A (waves) MAP TAP 2003-2004 The Electromagnetic Spectrum 2 MAP TAP 2003-2004 The Electromagnetic Spectrum 3 What is it? • The electromagnetic spectrum is the complete spectrum or continuum of light including radio waves, infrared, visible light, ultraviolet light, X- rays and gamma rays • An electromagnetic wave consists of electric and magnetic fields which vibrates thus making waves. MAP TAP 2003-2004 The Electromagnetic Spectrum 4 Waves • Properties of waves include speed, frequency and wavelength • Speed (s), frequency (f) and wavelength (l) are related in the formula l x f = s • All light travels at a speed of 3 s 108 m/s in a vacuum MAP TAP 2003-2004 The Electromagnetic Spectrum 5 Wavelength, Frequency and Energy • Since all light travels at the same speed, wavelength and frequency have an indirect relationship. • Light with a short wavelength will have a high frequency and light with a long wavelength will have a low frequency. • Light with short wavelengths has high energy and long wavelength has low energy MAP TAP 2003-2004 The Electromagnetic Spectrum 6 MAP TAP 2003-2004 The Electromagnetic Spectrum 7 Radio waves • Low energy waves with long wavelengths • Includes FM, AM, radar and TV waves • Wavelengths of 10-1m and longer • Low frequency • Used in many -

Violet Red Bile Agar M049
Violet Red Bile Agar M049 Violet Red Bile Agar is selective medium used for the isolation, detection and enumeration of coli-aerogenes bacteria in water, milk, other dairy food products and also from clinical samples. Composition** Ingredients Gms / Litre Peptic digest of animal tissue 7.000 Yeast extract 3.000 Sodium chloride 5.000 Bile salts mixture 1.500 Lactose 10.000 Neutral red 0.030 Crystal violet 0.002 Agar 15.000 Final pH ( at 25°C) 7.4±0.2 **Formula adjusted, standardized to suit performance parameters Directions Suspend 41.53 grams in 1000 ml distilled water. Heat with stirring to boiling to dissolve the medium completely. DO NOT AUTOCLAVE. Cool to 45°C and pour into sterile Petri plates containing the inoculum. If desired, the medium can be sterilized by autoclaving at 15 lbs pressure at 15lbs pressure (121°C) for 15 minutes. Principle And Interpretation The coliform group consists of several genera of bacteria belonging to the family Enterobacteriaceae . The historical definition of this group has been based on the method used for detection i.e. lactose fermentation. This group is defined as all aerobic and facultative anaerobic, gram-negative, non-spore-forming rod shaped bacteria that ferment lactose with gas and acid formation within 48 hour at 35°C (1, 2). Examination of foods, ingredients and raw materials, for the presence of marker groups such as coliforms is the one of the common tests. Violet Red Bile Agar, a modification of MacConkeys original formulation (3) is used for the enumeration of coli-aerogenes bacterial group. -

Electromagnetic Spectrum
Electromagnetic Spectrum Why do some things have colors? What makes color? Why do fast food restaurants use red lights to keep food warm? Why don’t they use green or blue light? Why do X-rays pass through the body and let us see through the body? What has the radio to do with radiation? What are the night vision devices that the army uses in night time fighting? To find the answers to these questions we have to examine the electromagnetic spectrum. FASTER THAN A SPEEDING BULLET MORE POWERFUL THAN A LOCOMOTIVE These words were used to introduce a fictional superhero named Superman. These same words can be used to help describe Electromagnetic Radiation. Electromagnetic Radiation is like a two member team racing together at incredible speeds across the vast regions of space or flying from the clutches of a tiny atom. They travel together in packages called photons. Moving along as a wave with frequency and wavelength they travel at the velocity of 186,000 miles per second (300,000,000 meters per second) in a vacuum. The photons are so tiny they cannot be seen even with powerful microscopes. If the photon encounters any charged particles along its journey it pushes and pulls them at the same frequency that the wave had when it started. The waves can circle the earth more than seven times in one second! If the waves are arranged in order of their wavelength and frequency the waves form the Electromagnetic Spectrum. They are described as electromagnetic because they are both electric and magnetic in nature. -

W Aves SCIENCE: Electromagnetic Spectrum
Keyword Definition Key facts to remember: electromagneti A group of waves that all travel at the same speed in All EM (electromagnetic) waves are transverse waves. c waves a vacuum, and are all transverse. frequency The number of vibrations (or the number of waves) Al l EM waves travel at the same speed (velocity) through a vacuum per second. One hertz (Hz) is one wave per second. (space) at 300 million m/s. infrared (IR) EM radiation that has a longer wavelength than EM waves are grouped based on their wavelengths and frequency. visible. We can feel infrared radiation as warmth. There are 7 basic EM waves. Radio waves, microwaves, infrared, visible light, UV, Xrays , gamma waves. SCIENCE: Waves interface The boundary between two materials. KS4 : AutumnKS4 Term KS4 : AutumnKS4 Term Our eyes can only detect a small part of this spectrum –visible light. refraction The change in direction when a wave goes from one medium to another. Different colours of light have different wavelengths from longest to transverse A wave in which the vibrations are at right angles to shortest: red, orange, yellow, green, blue, indigo, violet. (ROYGBIV) wave the direction the wave is travelling. or pneumonic; Richard Of York Gave Battle In Vain) ultraviolet (UV) EM radiation that has a shorter wavelength than visible light. Used to detect forged bank notes. vacuum A place where there is no matter at all. visible light Electromagnetic waves that can be detected by the human eye. gamma rays Electromagnetic radiation with the shortest SCIENCE: Electromagnetic Spectrum Electromagnetic SCIENCE: wavelengths and highest frequencies. -

Color Chart Colorchart
Color Chart AMERICANA ACRYLICS Snow (Titanium) White White Wash Cool White Warm White Light Buttermilk Buttermilk Oyster Beige Antique White Desert Sand Bleached Sand Eggshell Pink Chiffon Baby Blush Cotton Candy Electric Pink Poodleskirt Pink Baby Pink Petal Pink Bubblegum Pink Carousel Pink Royal Fuchsia Wild Berry Peony Pink Boysenberry Pink Dragon Fruit Joyful Pink Razzle Berry Berry Cobbler French Mauve Vintage Pink Terra Coral Blush Pink Coral Scarlet Watermelon Slice Cadmium Red Red Alert Cinnamon Drop True Red Calico Red Cherry Red Tuscan Red Berry Red Santa Red Brilliant Red Primary Red Country Red Tomato Red Naphthol Red Oxblood Burgundy Wine Heritage Brick Alizarin Crimson Deep Burgundy Napa Red Rookwood Red Antique Maroon Mulberry Cranberry Wine Natural Buff Sugared Peach White Peach Warm Beige Coral Cloud Cactus Flower Melon Coral Blush Bright Salmon Peaches 'n Cream Coral Shell Tangerine Bright Orange Jack-O'-Lantern Orange Spiced Pumpkin Tangelo Orange Orange Flame Canyon Orange Warm Sunset Cadmium Orange Dried Clay Persimmon Burnt Orange Georgia Clay Banana Cream Sand Pineapple Sunny Day Lemon Yellow Summer Squash Bright Yellow Cadmium Yellow Yellow Light Golden Yellow Primary Yellow Saffron Yellow Moon Yellow Marigold Golden Straw Yellow Ochre Camel True Ochre Antique Gold Antique Gold Deep Citron Green Margarita Chartreuse Yellow Olive Green Yellow Green Matcha Green Wasabi Green Celery Shoot Antique Green Light Sage Light Lime Pistachio Mint Irish Moss Sweet Mint Sage Mint Mint Julep Green Jadeite Glass Green Tree Jade -

Original Or Non-Original Expressive Art Guide
Expressive Art Oregon 4-H Tip Sheet OriginalArts or Non-original Art Guide Does the work use design components created by someone else? (patterns, drawings, recognizable pictures or photos, stencils, stamps, pre-shaped forms) NO YES Original Art Division Non-original Art Division Member applies the elements and principles of design Member applies the elements and principles of to create work that is entirely their own design to create work that may incorporate pieces designed or created by others. Pre-designed component must not be the total design Drawing & Sketching (by technique) Drawing/Shading Techniques • Line Drawing Uses drawing, shading, texturing and/or three Uses any drawing medium that can make a distinct line dimensional shaping techniques with the aid of partial Examples include pencil, colored pencil, scratch art, pen and photographs, line drawings, tracing or stenciling that ink, felt tip markers the member did not create themselves Differences in lightness/darkness can be achieved by spacing Includes soft metal embossing, woodburning, scratch or thickness of distinct lines art, or drawing to complete or enhance a partial photo • Shaded Drawing of a subject Uses any drawing medium that can make varied differences in lightness and darkness without showing distinct lines Shading is not simply adding color, it is a technique that adds dimension or volume to the piece. It introduces degrees of darkness to render light and shadow. Examples include chalk, charcoal, pastels, pencil, and colored pencil • Line & Shaded Combination -

BWSR Featured Plant: Downy Yellow Violet
2019 June Plant of the Month BWSR Featured Plant Name: Downy yellow violet (Viola pubescens) Plant family: Violet (Violaceae) Downy yellow Downy yellow violet, AKA hairy yellow violets are an important early violet or smooth yellow violet, is food source for a short (4- to 12-inch-tall), native, pollinators. Fine hairs along the herbaceous perennial that blooms in rounded teeth woodlands, gardens edge of the leaf are a distinguishing and shady areas Plant Stats feature. Brown lines starting in April. It STATEWIDE on the flower petals provides an early lead pollinators to WETLAND nectar and pollen. splash of color and INDICATOR Photo Credits: important early STATUS: FACU Heather Holm season nectar and PRIMARY USES: pollen. Like other Ground cover, shade/pollinator Viola species, this gardens, edibles, plant produces both woodland showy, open cross- restorations pollinating flowers at the top of the plant, and fully closed, self-pollinating flowers that may be found aboveground or underground. The showy flowers bloom before trees leaf out. The closed flowers bloom once the tree canopy leafs out. Planting Recommendations Range Downy yellow violets and can be used as Downy yellow violet is found may not be as an alternative to turf throughout Minnesota. Records aggressive as some grass, along paths and exist in all but a handful of other violets in a woodland borders, counties. It is mostly found east garden, but will spread and can be mixed with of the Missouri River, with a few over time in ideal other short woodland records west of the Missouri. conditions — part plants such as sedges, Its range stretches into New shade and medium to anemones and wild England and north into central dry soils. -

Darkness and Light: the Day of the Lordi
BIBLICAL PROPHECY—THINGS TO COME Darkness and light: The Day of the Lordi Now, brothers, about times and dates we do not need to write to you, for you know very well that the day of the Lord will come like a thief in the night. While people are saying, "Peace and safety," destruction will come on them suddenly, as labor pains on a pregnant woman, and they will not escape. But you, brothers, are not in darkness so that this day should surprise you like a thief. You are all sons of the light and sons of the day. We do not belong to the night or to the darkness. (1 Thessalonians 5:1-5) In these verses believers are called ―brothers.‖ Those who are saying, ―Peace and safety,‖ are unbelievers. God is reminding the Thessalonians that unbelievers will not escape judgment in the ―Day of the Lord.‖ However, believers are not in darkness, they are ―sons of the light,‖ sons of faith in Christ, and can look back on the accomplished salvation of Christ, which fulfilled Old Testament promises. They can look forward to the second coming of Christ, in the Day of the Lord, which consummates all of God’s prophecy/promises. The Day of the Lord was the high hope and the far-off goal of the Old Testament. It was, that toward which, the entire Old Testament program of God was moving. Everything in time and creation looked forward to and moved toward that day. The Old Testament era closed without it being realized, and up to today the Day of the Lord has not yet come.