Axert , a New “Triptan”
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW
Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW Name of Medicine / Trimipramine (Surmontil®) Class (generic and brand) Licensed indication(s) Treatment of depressive illness, especially where sleep disturbance, anxiety or agitation are presenting symptoms. Sleep disturbance is controlled within 24 hours and true antidepressant action follows within 7 to 10 days. Licensed dose(s) Adults: For depression 50-75 mg/day initially increasing to 150-300 mg/day in divided doses or one dose at night. The maintenance dose is 75-150 mg/day. Elderly: 10-25 mg three times a day initially. The initial dose should be increased with caution under close supervision. Half the normal maintenance dose may be sufficient to produce a satisfactory clinical response. Children: Not recommended. Purpose of Document To review information currently available on this class of medicines, give guidance on potential use and assign a prescribing classification http://www.cambsphn.nhs.uk/CJPG/CurrentDrugClassificationTable.aspx Annual cost (FP10) 10mg three times daily: £6,991 25mg three times daily: £7,819 150mg daily: £7,410 300mg daily: £14,820 Alternative Treatment Options within Class Tricyclic Annual Cost CPCCG Formulary Classification Antidepressant (FP10) Amitriptyline (75mg) Formulary £36 Lofepramine (140mg) Formulary £146 Imipramine (75mg) Non-formulary £37 Clomipramine (75mg) Non-formulary £63 Trimipramine (75mg). TBC £7,819 Nortriptyline (75mg) Not Recommended (pain) £276 Doxepin (150mg) TBC £6,006 Dosulepin (75mg) Not Recommended (NICE DO NOT DO) £19 Dosages are based on possible maintenance dose and are not equivalent between medications Recommendation It is recommended to Cambridgeshire and Peterborough CCG JPG members and through them to local NHS organisations that the arrangements for use of trimipramine are in line with restrictions agreed locally for drugs designated as NOT RECOMMENDED:. -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

Psychotropic Medications Judicial Reference Guide
PSYCHOTROPIC MEDICATIONS JUDICIAL REFERENCE GUIDE (Revised Edition 7/15/10) PSYCHOTROPIC MEDICATIONS JUDICIAL REFERENCE GUIDE FIRST EDITION THE STEERING COMMITTEE ON FAMILIES AND CHILDREN IN THE COURT Distributed by Florida Supreme Court 500 South Duval Street Tallahassee, FL 32399-1900 (850) 488-0125 INTRODUCTION One of the toughest challenges facing our dependency courts is the mental health of our children. “In July 2003, the Florida Statewide Advocacy Council published a Red Item Report finding 55% of foster children…in the state of Florida had been put on powerful mind altering psychotropic drugs.”1 In order to assist in this regard, the Psychotherapeutic Medication Subcommittee of the Steering Committee on Families and Children in the Court of the Supreme Court of Florida compiled this resource guide to help judges have a better understanding of psychotropic medications and their interaction with other drugs and with mental health disorders. Recently, the tragic case of Gabriel Myers in 2009 highlighted the fact that a number of child deaths were linked to the off label use of anti-psychotic medications. This is of special concern to Dependency Judges who are ultimately responsible for children in Florida’s Foster Care system. The researchers used publically available data from the internet, FDA manufactures’ published guidelines, publically available non-copyrighted articles and Dr. Brenda Thompson graciously prepared the Psychotropic Medication Chart. Special thanks to Dr. Brenda Thompson, the Honorable Herbert J. Baumann, the Honorable Ralph C. Stoddard, General Magistrate Tracy Ellis, Avron Bernstein, Selena Schoonover, Daniel Ringhoff, Jovasha Lang and to the Members of the Psychotherapeutic Medication Subcommittee. -

Decomposition Profile Data Analysis of Multiple Drug Effects Identifies
www.nature.com/scientificreports OPEN Decomposition profle data analysis of multiple drug efects identifes endoplasmic reticulum stress‑inducing ability as an unrecognized factor Katsuhisa Morita1,2, Tadahaya Mizuno1,2* & Hiroyuki Kusuhara1* Chemicals have multiple efects in biological systems. Because their on‑target efects dominate the output, their of‑target efects are often overlooked and can sometimes cause dangerous adverse events. Recently, we developed a novel decomposition profle data analysis method, orthogonal linear separation analysis (OLSA), to analyse multiple efects. In this study, we tested whether OLSA identifed the ability of drugs to induce endoplasmic reticulum (ER) stress as a previously unrecognized factor. After analysing the transcriptome profles of MCF7 cells treated with diferent chemicals, we focused on a vector characterized by well‑known ER stress inducers, such as ciclosporin A. We selected fve drugs predicted to be unrecognized ER stress inducers, based on their inducing ability scores derived from OLSA. These drugs actually induced X‑box binding protein 1 splicing, an indicator of ER stress, in MCF7 cells in a concentration‑dependent manner. Two structurally diferent representatives of the fve test compounds exhibited similar results in HepG2 and HuH7 cells, but not in PXB primary hepatocytes derived from human‑liver chimeric mice. These results indicate that our decomposition strategy using OLSA uncovered the ER stress‑inducing ability of drugs as an unrecognized efect, the manifestation of which depended on the background of the cells. Small compounds have the capacity to interact with multiple cellular proteins, thereby mediating multiple efects depending on their exposure to interacting proteins. It is quite difcult to identify the full range of efects of a new chemical, even for its developers, and some factors may be unrecognized. -

CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (Also Belong to Psychiatric Medication Category) • Codeine (In 222® Tablets, Tylenol® No
CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (also belong to psychiatric medication category) • codeine (in 222® Tablets, Tylenol® No. 1/2/3/4, Fiorinal® C, Benzodiazepines Codeine Contin, etc.) • heroin • alprazolam (Xanax®) • hydrocodone (Hycodan®, etc.) • chlordiazepoxide (Librium®) • hydromorphone (Dilaudid®) • clonazepam (Rivotril®) • methadone • diazepam (Valium®) • morphine (MS Contin®, M-Eslon®, Kadian®, Statex®, etc.) • flurazepam (Dalmane®) • oxycodone (in Oxycocet®, Percocet®, Percodan®, OxyContin®, etc.) • lorazepam (Ativan®) • pentazocine (Talwin®) • nitrazepam (Mogadon®) • oxazepam ( Serax®) Alcohol • temazepam (Restoril®) Inhalants Barbiturates • gases (e.g. nitrous oxide, “laughing gas”, chloroform, halothane, • butalbital (in Fiorinal®) ether) • secobarbital (Seconal®) • volatile solvents (benzene, toluene, xylene, acetone, naptha and hexane) Buspirone (Buspar®) • nitrites (amyl nitrite, butyl nitrite and cyclohexyl nitrite – also known as “poppers”) Non-Benzodiazepine Hypnotics (also belong to psychiatric medication category) • chloral hydrate • zopiclone (Imovane®) Other • GHB (gamma-hydroxybutyrate) • Rohypnol (flunitrazepam) CENTRAL NERVOUS SYSTEM STIMULANTS Amphetamines Caffeine • dextroamphetamine (Dexadrine®) Methelynedioxyamphetamine (MDA) • methamphetamine (“Crystal meth”) (also has hallucinogenic actions) • methylphenidate (Biphentin®, Concerta®, Ritalin®) • mixed amphetamine salts (Adderall XR®) 3,4-Methelynedioxymethamphetamine (MDMA, Ecstasy) (also has hallucinogenic actions) Cocaine/Crack -
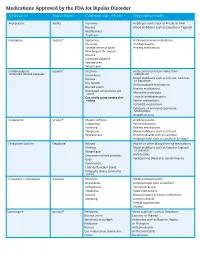
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common Side Effects May Interact With
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common side effects May interact with Aripiprazole Abilify® Insomnia Antidepressants such as Prozac or Paxil Nausea Mood stabilizers such as Equetro or Tegretol Restlessness Tiredness Asenapine Saphris® Sleepiness Antihypertensive medications Dizziness Antidepressants Strange sense of taste Anxiety medications Numbing of the mouth Nausea Increased appetite Feeling tired Weight gain Carbamazepine Equetro™ Dizziness Birth control pills (can make them extended release capsules Drowsiness ineffective) Mood stabilizers such as Lithium, Lamictal, Nausea or Depakote Dry mouth Anticonvulsant medications Blurred vision Anxiety medications Decreased white blood cell count Macrolide antibiotics Can rarely cause severe skin Tricyclic antidepressants rashes Cancer medications HIV/AIDS medications Cytotoxic or immunosuppressive Medications Grapefruit juice Cariprazine Vraylar® Muscle stiffness Antidepressants Indigestion Pain medications Vomiting Anxiety medications Sleepiness Mood stabilizers such as Lithium Restlessness Anticonvulsants such as Lamictal Antipsychotics such as Latuda or Seroquel Divalproex Sodium Depakote® Nausea Aspirin or other blood thinning medications Shaking Mood stabilizers such as Equetro,Tegretol, Weight gain or Lamictal Decrease in blood platelets Barbiturates Rash Cyclosporine (Neoral or Sandimmune) Pancreatitis Liver dysfunction (rare) Polycystic Ovary Syndrome (rare) Fluoxetine + Olanzapine Symbyax® Dizziness MAOI antidepressants Drowsiness Antipsychotics -

Trimipramine
PATIENT & CAREGIVER EDUCATION Trimipramine This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Surmontil [DSC] Brand Names: Canada APO-Trimipramine Warning Drugs like this one have raised the chance of suicidal thoughts or actions in children and young adults. The risk may be greater in people who have had these thoughts or actions in the past. All people who take this drug need to be watched closely. Call the doctor right away if signs like low mood (depression), nervousness, restlessness, grouchiness, panic attacks, or changes in mood or actions are new or worse. Call the doctor right away if any thoughts or actions of suicide occur. This drug is not approved for use in children. Talk with the doctor. Trimipramine 1/10 What is this drug used for? It is used to treat low mood (depression). What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have had a recent heart attack. If you have taken certain drugs for depression or Parkinson’s disease in the last 14 days. This includes isocarboxazid, phenelzine, tranylcypromine, selegiline, or rasagiline. Very high blood pressure may happen. If you are taking any of these drugs: Linezolid or methylene blue. This is not a list of all drugs or health problems that interact with this drug. -

World Health Organization Model List of Essential Medicines, 21St List, 2019
World Health Organizatio n Model List of Essential Medicines 21st List 2019 World Health Organizatio n Model List of Essential Medicines 21st List 2019 WHO/MVP/EMP/IAU/2019.06 © World Health Organization 2019 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. CIP data are available at http://apps.who.int/iris. -

Asis with Tropicamide and Phenylephrine on Intraocular Pressure
Adediji AK, et al., J Ophthalmic Clin Res 2019, 6: 049 DOI: 10.24966/OCR-8887/100049 HSOA Journal of Ophthalmology and Clinical Research Research Article line at all the follow up periods except at 45 minutes where it was Effects of Diagnostic Mydri- slightly higher (12.11±2.87mmHg). These differences in mean IOP change were statistically significant (p<0.05). At all the follow-up asis with Tropicamide and periods, 8.8%-14.6% of participant’s right eyes had large IOP ele- vations (>5mmHg but <10mmHg). Multiple linear regression anal- Phenylephrine on Intraocular ysis showed that pre dilatation IOPs were positively correlated to post dilatation IOPs. Pressure Conclusion: There is need to recheck IOP post dilatation prefera- Adediji AK, Adio AO and Fiebai B* bly at 45 minutes in all patients who have had diagnostic mydriasis to prevent damage to the optic nerve. Diagnostic mydriasis could Department of Ophthalmology, University of Port Harcourt Teaching Hospi- safely be done using small concentrations of tropicamide and phen- tal, Port Harcourt, Nigeria ylephrine. Keywords: Diagnostic mydriasis; Intraocular pressure; Mydriasis; Phenylephrine; Tropicamide Introduction Intraocular Pressure (IOP) is the tissue pressure within the eye which is determined by the balance between aqueous humor produc- tion and outflow, which under normal circumstances is nearly equal. The normal distribution of IOP within the general population is 11- 21mmHg [1]. Intraocular pressure can be affected by a range of fac- tors like time of the day, heartbeat, respiration, exercise, fluid intake, Abstract posture, blinking, eye movements, valsalva manoeuvres and med- ications; including those used for pupillary dilatation [2-4]. -
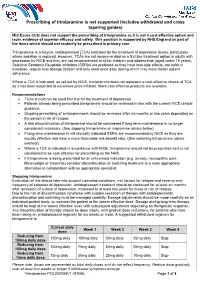
Prescribing of Trimipramine Is Not Supported (Includes Withdrawal and Cross Tapering Guides)
Prescribing of trimipramine is not supported (includes withdrawal and cross tapering guides) Mid Essex CCG does not support the prescribing of trimipramine as it is not a cost-effective option and lacks evidence of superior efficacy and safety. This position is supported by NHS England as part of the items which should not routinely be prescribed in primary care. Trimipramine is a tricyclic antidepressant (TCA) indicated for the treatment of depressive illness, particularly where sedation is required. However, TCAs are not recommended as a first line treatment option in adults with depression by NICE and they are not recommended at all for children and adolescents (aged under 18 years). Selective Serotonin Reuptake Inhibitors (SSRIs) are preferred as they have less side effects, are safer in overdose, require less dosage titration and only need once daily dosing which may mean better patient adherence. Where a TCA is indicated, as set out by NICE, trimipramine does not represent a cost-effective choice of TCA as it has been subjected to excessive price inflation. More cost effective products are available. Recommendations • TCAs should not be used first line for the treatment of depression. • Patients already being prescribed trimipramine should be reviewed in line with the current NICE clinical guidance. • Ongoing prescribing of antidepressants should be reviewed after six months or two years depending on the person’s risk of relapse. • A trial discontinuation of trimipramine should be considered if long-term maintenance is no longer considered necessary. (See stopping trimipramine or imipramine advice below) • If long-term maintenance is still clinically indicated SSRIs are recommended by NICE as they are equally effective and have a more favourable risk-benefit ratio. -

Appendix 1 BNF Codes of Drugs Lists Used to Define Exposure Groups
Appendix 1 BNF Codes of drugs lists used to define exposure groups. Anticholinergic antipsychotics d411. Chlorpromazine d412. Chlorpromazine d413. Chlorpromazine d414. Chlorpromazine d415. Chlorpromazine d41a. Chlorpromazine d41b. Chlorpromazine d41c. Chlorpromazine d41d. Chlorpromazine d4b1. Perphenazine d4e1. PROMAZINE d4ex. PROMAZINE d4g.. Thioridazine d4g1. Thioridazine d4g2. Thioridazine d4g3. Thioridazine d4g5. Thioridazine d4g7. Thioridazine d4gp. Thioridazine d4gt. Thioridazine d4gu. Thioridazine d4gv. Thioridazine d4gw. Thioridazine d4gz. Thioridazine d4h.. Trifluoperazine d4h1. Trifluoperazine d4h2. Trifluoperazine d4h3. Trifluoperazine d4h4. Trifluoperazine d4hs. Trifluoperazine d4ht. Trifluoperazine d4hu. Trifluoperazine d4hx. Trifluoperazine d4l2. CLOZAPINE d4r1. OLANZAPINE d4r3. OLANZAPINE d4r7. OLANZAPINE d4s1. QUETIAPINE d4s2. QUETIAPINE d4s3. QUETIAPINE d4s5. QUETIAPINE d4ss. QUETIAPINE d4sx. QUETIAPINE Tricyclic antidepressants d7... d71.. Amitriptyline d711. Amitriptyline d712. Amitriptyline d713. Amitriptyline d719. Amitriptyline d71a. Amitriptyline d71b. Amitriptyline d71c. Amitriptyline d71d. Amitriptyline d71e. Amitriptyline d71f. Amitriptyline d71u. Amitriptyline d71v. Amitriptyline d71w. Amitriptyline d71y. Amitriptyline d71z. Amitriptyline d73.. Clomipramine d731. Clomipramine d732. Clomipramine d733. Clomipramine d736. Clomipramine d73s. Clomipramine d73t. Clomipramine d73u. Clomipramine d73v. Clomipramine d73w. Clomipramine d73z. Clomipramine d75.. DOSULEPIN d751. DOSULEPIN d752. DOSULEPIN d755. DOSULEPIN d756. -

Queensland Health List of Approved Medicines
Queensland Health List of Approved Medicines Drug Form Strength Restriction abacavir * For use in accord with PBS Section 100 indications * oral liquid See above 20 mg/mL See above tablet See above 300 mg See above abacavir + lamivudine * For use in accord with PBS Section 100 indications * tablet See above 600 mg + 300 mg See above abacavir + lamivudine + * For use in accord with PBS Section 100 indications * zidovudine tablet See above 300 mg + 150 mg + 300 mg See above abatacept injection 250 mg * For use in accord with PBS Section 100 indications * abciximab (a) Interventional Cardiologists for complex angioplasty (b) Interventional and Neuro-interventional Radiologists for rescue treatment of thromboembolic events that occur during neuroendovascular procedures. * Where a medicine is not TGA approved, patients should be made fully aware of the status of the medicine and appropriate consent obtained * injection See above 10 mg/5 mL See above abiraterone For use by medical oncologists as per the PBS indications for outpatient and discharge use only tablet See above 250 mg See above 500 mg See above acamprosate Drug and alcohol treatment physicians for use with a comprehensive treatment program for alcohol dependence with the goal of maintaining abstinence. enteric tablet See above 333 mg See above acarbose For non-insulin dependent diabetics with inadequate control despite diet; exercise and maximal tolerated doses of other anti-diabetic agents tablet See above 50 mg See above 100 mg See above acetazolamide injection 500 mg tablet 250 mg acetic acid ear drops 3% 15mL solution 2% 100mL green 3% 1 litre 6% 1 Litre 6% 200mL Generated on: 30-Aug-2021 Page 1 of 142 Drug Form Strength Restriction acetylcysteine injection For management of paracetamol overdose 2 g/10 mL See above 6 g/30 mL See above aciclovir cream Infectious disease physicians, haematologists and oncologists 5% See above eye ointment For use on the advice of Ophthalmologists only.