Partial Purification and Characterization of an Ls-NADP: Isocitrate Oxidoreductase from Embryonic Chick Liver
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Altered Expression and Function of Mitochondrial Я-Oxidation Enzymes
0031-3998/01/5001-0083 PEDIATRIC RESEARCH Vol. 50, No. 1, 2001 Copyright © 2001 International Pediatric Research Foundation, Inc. Printed in U.S.A. Altered Expression and Function of Mitochondrial -Oxidation Enzymes in Juvenile Intrauterine-Growth-Retarded Rat Skeletal Muscle ROBERT H. LANE, DAVID E. KELLEY, VLADIMIR H. RITOV, ANNA E. TSIRKA, AND ELISA M. GRUETZMACHER Department of Pediatrics, UCLA School of Medicine, Mattel Children’s Hospital at UCLA, Los Angeles, California 90095, U.S.A. [R.H.L.]; and Departments of Internal Medicine [D.E.K., V.H.R.] and Pediatrics [R.H.L., A.E.T., E.M.G.], University of Pittsburgh School of Medicine, Magee-Womens Research Institute, Pittsburgh, Pennsylvania 15213, U.S.A. ABSTRACT Uteroplacental insufficiency and subsequent intrauterine creased in IUGR skeletal muscle mitochondria, and isocitrate growth retardation (IUGR) affects postnatal metabolism. In ju- dehydrogenase activity was unchanged. Interestingly, skeletal venile rats, IUGR alters skeletal muscle mitochondrial gene muscle triglycerides were significantly increased in IUGR skel- expression and reduces mitochondrial NADϩ/NADH ratios, both etal muscle. We conclude that uteroplacental insufficiency alters of which affect -oxidation flux. We therefore hypothesized that IUGR skeletal muscle mitochondrial lipid metabolism, and we gene expression and function of mitochondrial -oxidation en- speculate that the changes observed in this study play a role in zymes would be altered in juvenile IUGR skeletal muscle. To test the long-term morbidity associated with IUGR. (Pediatr Res 50: this hypothesis, mRNA levels of five key mitochondrial enzymes 83–90, 2001) (carnitine palmitoyltransferase I, trifunctional protein of -oxi- dation, uncoupling protein-3, isocitrate dehydrogenase, and mi- Abbreviations tochondrial malate dehydrogenase) and intramuscular triglycer- CPTI, carnitine palmitoyltransferase I ides were quantified in 21-d-old (preweaning) IUGR and control IUGR, intrauterine growth retardation rat skeletal muscle. -

Isocitrate Dehydrogenase 1 (NADP+) (I5036)
Isocitrate Dehydrogenase 1 (NADP+), human recombinant, expressed in Escherichia coli Catalog Number I5036 Storage Temperature –20 °C CAS RN 9028-48-2 IDH1 and IDH2 have frequent genetic alterations in EC 1.1.1.42 acute myeloid leukemia4 and better understanding of Systematic name: Isocitrate:NADP+ oxidoreductase these mutations may lead to an improvement of (decarboxylating) individual cancer risk assessment.6 In addition other studies have shown loss of IDH1 in bladder cancer Synonyms: IDH1, cytosolic NADP(+)-dependent patients during tumor development suggesting this may isocitrate dehydrogenase, isocitrate:NADP+ be involved in tumor progression and metastasis.7 oxidoreductase (decarboxylating), Isocitric Dehydrogenase, ICD1, PICD, IDPC, ICDC, This product is lyophilized from a solution containing oxalosuccinate decarboxylase Tris-HCl, pH 8.0, with trehalose, ammonium sulfate, and DTT. Product Description Isocitrate dehydrogenase (NADP+) [EC 1.1.1.42] is a Purity: ³90% (SDS-PAGE) Krebs cycle enzyme, which converts isocitrate to a-ketoglutarate. The flow of isocitrate through the Specific activity: ³80 units/mg protein glyoxylate bypass is regulated by phosphorylation of isocitrate dehydrogenase, which competes for a Unit definition: 1 unit corresponds to the amount of 1 common substrate (isocitrate) with isocitrate lyase. enzyme, which converts 1 mmole of DL-isocitrate to The activity of the enzyme is dependent on the a-ketoglutarate per minute at pH 7.4 and 37 °C (NADP formation of a magnesium or manganese-isocitrate as cofactor). The activity is measured by observing the 2 complex. reduction of NADP to NADPH at 340 nm in the 7 presence of 4 mM DL-isocitrate and 2 mM MnSO4. -

Pro-Aging Effects of Xanthine Oxidoreductase Products
antioxidants Review Pro-Aging Effects of Xanthine Oxidoreductase Products , , Maria Giulia Battelli y , Massimo Bortolotti y , Andrea Bolognesi * z and Letizia Polito * z Department of Experimental, Diagnostic and Specialty Medicine-DIMES, Alma Mater Studiorum, University of Bologna, Via San Giacomo 14, 40126 Bologna, Italy; [email protected] (M.G.B.); [email protected] (M.B.) * Correspondence: [email protected] (A.B.); [email protected] (L.P.); Tel.: +39-051-20-9-4707 (A.B.); +39-051-20-9-4729 (L.P.) These authors contributed equally. y Co-last authors. z Received: 22 July 2020; Accepted: 4 September 2020; Published: 8 September 2020 Abstract: The senescence process is the result of a series of factors that start from the genetic constitution interacting with epigenetic modifications induced by endogenous and environmental causes and that lead to a progressive deterioration at the cellular and functional levels. One of the main causes of aging is oxidative stress deriving from the imbalance between the production of reactive oxygen (ROS) and nitrogen (RNS) species and their scavenging through antioxidants. Xanthine oxidoreductase (XOR) activities produce uric acid, as well as reactive oxygen and nitrogen species, which all may be relevant to such equilibrium. This review analyzes XOR activity through in vitro experiments, animal studies and clinical reports, which highlight the pro-aging effects of XOR products. However, XOR activity contributes to a regular level of ROS and RNS, which appears essential for the proper functioning of many physiological pathways. This discourages the use of therapies with XOR inhibitors, unless symptomatic hyperuricemia is present. -

RESEARCH COMMUNICATION HADHA Is a Potential Predictor Of
HADHA is a Potential Predictor of the Response to Platinum-based Chemotherapy RESEARCH COMMUNICATION HADHA is a Potential Predictor of Response to Platinum-based Chemotherapy for Lung Cancer Taihei Kageyama1, Ryo Nagashio1, 2, Shinichiro Ryuge 3, Toshihide Matsumoto1,5, Akira Iyoda4, Yukitoshi Satoh4, Noriyuki Masuda3, Shi-Xu Jiang5, Makoto Saegusa5, Yuichi Sato1, 2* Abstract To identify a cisplatin resistance predictor to reduce or prevent unnecessary side effects, we firstly established four cisplatin-resistant sub-lines and compared their protein profiles with cisplatin-sensitive parent lung cancer cell lines using two-dimensional gel electrophoresis. Between the cisplatin-resistant and -sensitive cells, a total of 359 protein spots were differently expressed (>1.5 fold), and 217 proteins (83.0%) were identified. We focused on a mitochondrial protein, hydroxyl-coenzyme A dehydrogenase/3-ketoacyl-coenzyme A thiolase/enoyl-coenzyme A hydratase alpha subunit (HADHA), which was increased in all cisplatin-resistant cells. Furthermore, pre- treated biopsy specimens taken from patients who showed resistance to platinum-based treatment showed a significantly higher positive rate for HADHA in all cases (p=0.00367), including non-small cell lung carcinomas (p=0.002), small-cell lung carcinomas (p=0.038), and adenocarcinomas (p=0.008). These results suggest that the expression of HADHA may be a useful marker to predict resistance to platinum-based chemotherapy in patients with lung cancer. Keywords: Cisplatin - HADHA - lung cancer - two-dimensional gel electrophoresis Asian Pacific J Cancer Prev, 12, 3457-3463 Introduction cisplatin resistance rose due to a decrease of blood flow in the tumor and increased DNA repair (Stewart, 2007), Lung cancer is the leading cause of cancer-related the mechanisms underlying cisplatin resistance have not death in the world, and the five-year overall survival rate yet been clarified, and an effective cisplatin resistance is still below 16% (Jemal et al., 2009). -

Glyoxysomal Malate Dehydrogenase from Watermelon Is Synthesized
Proc. Nati. Acad. Sci. USA Vol. 87, pp. 5773-5777, August 1990 Botany Glyoxysomal malate dehydrogenase from watermelon is synthesized with an amino-terminal transit peptide (isoenzymes/organelle/Citrulus vulgaris/polymerase chain reaction) CHRISTINE GIETL* Institute of Botany, Technical University of Munich, Arcisstrasse 16, D-8000 Munich 2, Federal Republic of Germany; and Department of Physiology, Carlsberg Laboratory, Gamle Carlsberg Vej 10, DK-2500 Copenhagen Valby, Denmark Communicated by Diter von Wettstein, May 11, 1990 (receivedfor review March 5, 1990) ABSTRACT The isolation and sequence of a cDNA clone and peroxisomes are seen between mitochondria and chlo- encoding the complete glyoxysomal malate dehydrogenase roplasts (2). As in plants, mammalian peroxisomes contain [gMDH; (S)-malate:NAD+ oxidoreductase, EC 1.1.1.37] of enzymes involved in the production and degradation ofH202; watermelon cotyledons are presented. Partial cDNA clones in trypanosomes, glycolysis is sequestered into microbodies were synthesized in a three part strategy, taking advantage of called glycosomes (3). All microbodies studied contain en- the polymerase chain reaction technology with oligonucleotides zymes for (3-oxidation and a specific spectrum of other based on directly determined amino acid sequences. Subse- enzymes. It is further characteristic that microbody enzyme quently, the complete done for gMDH was synthesized with a activities are also present in other cell compartments. Gly- sense primer corresponding to the nucleotide sequence of the oxysomes as well as mitochondria contain MDH, citrate N-terminal end of pre-gMDIH and an antisense primer corre- synthase, and enzymes for 83-oxidation (4). The organelle- sponding to the adenylylation site found in the mRNA. -

Cholic Acid for Treating Inborn Errors of Primary Bile Acid Synthesis NHS England Unique Reference Number URN1623 / NICE ID004
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Clinical evidence review of cholic acid for treating inborn errors of primary bile acid synthesis NHS England unique reference number URN1623 / NICE ID004 Prepared by: NICE on behalf of NHS England Specialised Commissioning About this clinical evidence review Clinical evidence reviews are a summary of the best available evidence for a single technology within a licensed indication, for commissioning by NHS England. The clinical evidence review supports NHS England in producing clinical policies but is not NICE guidance or advice. Summary This evidence review considers cholic acid (Laboratoires CTRS [Orphacol] and Retrophin Europe Ltd [Kolbam]) for treating inborn errors of primary bile acid NICE clinical evidence review of cholic acid for treating inborn errors of primary bile acid synthesis Page 1 of 72 NHS URN1623 NICE ID004 synthesis caused by the following enzyme deficiencies in people aged 1 month and over: 3-beta-hydroxy-delta5-C27-steroid oxidoreductase (3beta-HSD) delta4-3-oxosteroid-5-beta reductase (5beta-reductase) 2- (or alpha-) methylacyl-CoA racemase (AMACR) sterol 27-hydroxylase (presenting as cerebrotendinous xanthomatosis [CTX]) cholesterol 7alpha-hydroxylase (CYP7A1). Inborn errors of primary bile acid synthesis are rare genetic conditions in which enzyme deficiencies prevent the liver from converting cholesterol in the body to bile acids (such as cholic acid and chenodeoxycholic acid). This results in the liver producing high concentrations of atypical (or ‘unusual’) bile acids and intermediary metabolites (some of which are toxic to the liver) in an attempt to establish a normal bile acid pool. Accumulation of potentially toxic atypical bile acids and metabolites, and reduced flow of bile acids may cause liver injury. -
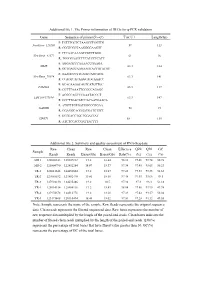
Additional File 1
Additional file 1. The Primer information of DEGs for q-PCR validation Gene Sequence of primer(5'→3') Tm(℃) Length(bp) F: TGTTTGCTCTAAGCCTGGTTG NewGene_126260 59 113 R: CGGTCGCTAAGGGGAAGTT F: CTCAACAAAGCCGTCTGGG NewGene_41572 61 96 R: TGGGGAATCTTCATCCTCATT F: AGGAGCCCAAAACCGAAGA MME 63.3 184 R: GCTGACCAAGAAGTACCGTATGT F: AAAGCCCTTCAGTCAGCACG NewGene_70974 63.3 141 R: CCAGTCACAAGCAGCAAACC F: GCACAAGGCAGTCATGTTGC FAM43A 63.3 117 R: CGTTTAAATTCCGCCAGAGC F: ACGGCAGCCCAAATACCCT LOC108177184 63.3 147 R: GCCTTGACATCCACAATGAACA F: ATGTTTGTGATGGGCGTGAA GAPDH 58 94 R: GGAGGCAGGGATGATGTTCT F: GCTGACCTGCTGGATTAT HPRT1 58 135 R: ATCTCCACCGATTACTTT Additional file 2. Summary and quality assessment of RNA-Seq data Raw Clean Raw Clean Effective Q20 Q30 GC Sample Reads Reads Bases(Gb) Bases(Gb) Rate(%) (%) (%) (%) MR-1 128008342 125607312 19.2 18.84 98.12 97.46 93.74 50.78 MR-2 125804790 122452284 18.87 18.37 97.34 97.45 93.65 50.23 TR-1 128011648 124452604 19.2 18.67 97.22 97.33 93.35 51.65 TR-2 127085532 123882470 19.06 18.58 97.48 97.45 93.66 49.5 TR-3 127994130 124635486 19.2 18.7 97.38 97.3 93.3 51.18 YR-1 128014314 125504116 19.2 18.83 98.04 97.56 93.95 49.98 YR-2 127974678 124512778 19.2 18.68 97.29 97.42 93.57 50.04 YR-3 123173408 120110494 18.48 18.02 97.51 97.28 93.32 49.55 Note: Sample represents the name of the sample. Raw Reads represents the original sequence data. Clean reads represents the filtered sequenced data. Raw bases represents the number of raw sequence data multiplied by the length of the paired-end reads. -

Bioactivity of Curcumin on the Cytochrome P450 Enzymes of the Steroidogenic Pathway
International Journal of Molecular Sciences Article Bioactivity of Curcumin on the Cytochrome P450 Enzymes of the Steroidogenic Pathway Patricia Rodríguez Castaño 1,2, Shaheena Parween 1,2 and Amit V Pandey 1,2,* 1 Pediatric Endocrinology, Diabetology, and Metabolism, University Children’s Hospital Bern, 3010 Bern, Switzerland; [email protected] (P.R.C.); [email protected] (S.P.) 2 Department of Biomedical Research, University of Bern, 3010 Bern, Switzerland * Correspondence: [email protected]; Tel.: +41-31-632-9637 Received: 5 September 2019; Accepted: 16 September 2019; Published: 17 September 2019 Abstract: Turmeric, a popular ingredient in the cuisine of many Asian countries, comes from the roots of the Curcuma longa and is known for its use in Chinese and Ayurvedic medicine. Turmeric is rich in curcuminoids, including curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Curcuminoids have potent wound healing, anti-inflammatory, and anti-carcinogenic activities. While curcuminoids have been studied for many years, not much is known about their effects on steroid metabolism. Since many anti-cancer drugs target enzymes from the steroidogenic pathway, we tested the effect of curcuminoids on cytochrome P450 CYP17A1, CYP21A2, and CYP19A1 enzyme activities. When using 10 µg/mL of curcuminoids, both the 17α-hydroxylase as well as 17,20 lyase activities of CYP17A1 were reduced significantly. On the other hand, only a mild reduction in CYP21A2 activity was observed. Furthermore, CYP19A1 activity was also reduced up to ~20% of control when using 1–100 µg/mL of curcuminoids in a dose-dependent manner. Molecular docking studies confirmed that curcumin could dock onto the active sites of CYP17A1, CYP19A1, as well as CYP21A2. -

Flavonoids, Cinnamic Acid Derivatives As Inhibitors of 17(-Hydroxysteroid
Flavonoids, cinnamic acid derivatives as inhibitors of 17(-hydroxysteroid dehydrogenase type 1 Petra Brožič, Petra Kocbek, Matej Sova, Julijana Kristl, Stefan Martens, Jerzy Adamski, Stanislav Gobec, Tea Lanišnik Rižner To cite this version: Petra Brožič, Petra Kocbek, Matej Sova, Julijana Kristl, Stefan Martens, et al.. Flavonoids, cinnamic acid derivatives as inhibitors of 17(-hydroxysteroid dehydrogenase type 1. Molecular and Cellular Endocrinology, Elsevier, 2009, 301 (1-2), pp.229. 10.1016/j.mce.2008.09.004. hal-00532074 HAL Id: hal-00532074 https://hal.archives-ouvertes.fr/hal-00532074 Submitted on 4 Nov 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Title: Flavonoids, cinnamic acid derivatives as inhibitors of 17(-hydroxysteroid dehydrogenase type 1 Authors: Petra Broziˇ c,ˇ Petra Kocbek, Matej Sova, Julijana Kristl, Stefan Martens, Jerzy Adamski, Stanislav Gobec, Tea Lanisnikˇ Riznerˇ PII: S0303-7207(08)00398-5 DOI: doi:10.1016/j.mce.2008.09.004 Reference: MCE 6970 To appear in: Molecular and Cellular Endocrinology Received date: 30-6-2008 Revised date: 29-8-2008 Accepted date: 1-9-2008 Please cite this article as: Broziˇ c,ˇ P., Kocbek, P., Sova, M., Kristl, J., Martens, S., Adamski, J., Gobec, S., Rizner,ˇ T.L., Flavonoids, cinnamic acid derivatives as inhibitors of 17(-hydroxysteroid dehydrogenase type 1, Molecular and Cellular Endocrinology (2008), doi:10.1016/j.mce.2008.09.004 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Structural Basis for the Electron Transfer from an Open Form of NADPH-Cytochrome P450 Oxidoreductase to Heme Oxygenase
Structural basis for the electron transfer from an open form of NADPH-cytochrome P450 oxidoreductase to heme oxygenase Masakazu Sugishimaa,1, Hideaki Satoa, Yuichiro Higashimotob, Jiro Haradaa, Kei Wadac, Keiichi Fukuyamad, and Masato Noguchia,1 aDepartment of Medical Biochemistry and bDepartment of Chemistry, Kurume University School of Medicine, Kurume, Fukuoka 830-0011, Japan; cOrganization for Promotion of Tenure Track, University of Miyazaki, Kiyotake, Miyazaki 889-1692, Japan; and dDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan Edited by Thomas C. Pochapsky, Brandeis University, Waltham, MA, and accepted by the Editorial Board January 14, 2014 (received for review November 26, 2013) NADPH-cytochrome P450 oxidoreductase (CPR) supplies electrons hardly conceivable that the redox partners such as cytochrome to various heme proteins including heme oxygenase (HO), which is P450 could approach closely enough to FMN for intermolecular a key enzyme for heme degradation. Electrons from NADPH flow electron transfer to occur. Site-directed mutagenesis analysis first to flavin adenine dinucleotide, then to flavin mononucleotide demonstrated that the acidic clusters (Asp207-Asp208-Asp209 (FMN), and finally to heme in the redox partner. For electron trans- and Glu213-Glu214-Asp215 in rat CPR) near FMN are involved fer from CPR to its redox partner, the ‘‘closed-open transition’’ of in the association with cytochrome P450s (4), but these clusters CPR is indispensable. Here, we demonstrate that a hinge-short- were covered by the FAD domain in the closed conformation ened CPR variant, which favors an open conformation, makes a sta- (3). Therefore, a large conformational change in CPR is expec- ble complex with heme–HO-1 and can support the HO reaction, ted to take place for smooth interaction between CPR and its although its efficiency is extremely limited. -

Significance to Female Reproductive Physiology1
[CANCER RESEARCH (SUPPL.) 42, 3342s-3344s, August 1982] 0008-5472/82/0042-OOOOS02.00 Biochemistry of Aromatase: Significance to Female Reproductive Physiology1 Kenneth J. Ryan Laboratory of Human Reproduction and Reproductive Biology, Department of Obstetrics and Gynecology. Harvard Medical School, Boston, Massachusetts 02115 Abstract adrenal, fetal liver, skin, fat, breast, and bone marrow and, at least in the brain, ovary, and placenta, the enzyme is present The formation of estrogens in mammals via aromatase in in many different vertebrate species (3, 5). In the ovary, aro volves the relatively unique capacity to form an aromatic ring matase is the final step in a multienzyme system that synthe de novo in contrast to most other aromatic substances (essen sizes estrogen from acetate and cholesterol while, in most tial amino acids) which are obtained only in the diet. The other tissues, aromatase acts on preformed plasma precursor reaction is the only example of a cytochrome P-450 system androgens secreted by the adrenal or gonad (16). For many which resides in both the mitochondrial and microsomal frac species, the source of the androgen substrate for placental tions of the cell. It occurs widely throughout the body in diverse aromatase is the fetal adrenal (14). tissues and functions via both de novo synthesis and transfor Aromatase is unique in that it resides in both the microsomal mation of prehormones (androstenedione and testosterone). It and mitochondrial fractions of cells and as far as can be is found widely in animal species in both the brain and gonads determined is the same type of enzyme in both cellular subfrac- even in phylogenetically primitive species. -

Role of NAD+-Dependent Malate Dehydrogenase in the Metabolism of Methylomicrobium Alcaliphilum 20Z and Methylosinus Trichosporium Ob3b
Microorganisms 2015, 3, 47-59; doi:10.3390/microorganisms3010047 OPEN ACCESS microorganisms ISSN 2076-2607 www.mdpi.com/journal/microorganisms Article Role of NAD+-Dependent Malate Dehydrogenase in the Metabolism of Methylomicrobium alcaliphilum 20Z and Methylosinus trichosporium OB3b Olga N. Rozova 1, Valentina N. Khmelenina 1,*, Ksenia A. Bocharova 2, Ildar I. Mustakhimov 2 and Yuri A. Trotsenko 1,2 1 Laboratory of Methylotrophy, Skryabin Institute of Biochemistry and Physiology of Microorganisms, RAS, Prospect Nauki 5, Pushchino 142290, Russia; E-Mails: [email protected] (O.N.R.); [email protected] (Y.A.T.) 2 Department of Microbiology and Biotechnology, Pushchino State Institute of Natural Sciences, Prospect Nauki 3, Pushchino 142290, Russia; E-Mails: [email protected] (K.A.B.); [email protected] (I.I.M.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +7-4967-318672; Fax: +7-4959-563370. Academic Editors: Marina G. Kalyuzhnaya and Ludmila Chistoserdova Received: 30 December 2014 / Accepted: 5 February 2015 / Published: 27 February 2015 Abstract: We have expressed the L-malate dehydrogenase (MDH) genes from aerobic methanotrophs Methylomicrobium alcaliphilum 20Z and Methylosinus trichosporium OB3b as his-tagged proteins in Escherichia coli. The substrate specificities, enzymatic kinetics and oligomeric states of the MDHs have been characterized. Both MDHs were NAD+-specific and thermostable enzymes not affected by metal ions or various organic metabolites. The MDH from M. alcaliphilum 20Z was a homodimeric (2 × 35 kDa) enzyme displaying nearly equal reductive (malate formation) and oxidative (oxaloacetate formation) activities and higher affinity to malate (Km = 0.11 mM) than to oxaloacetate (Km = 0.34 mM).