Propagation of Wheat Fusarium Wilt in Morocco
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cadastre Des Autorisations TPV Page 1 De
Cadastre des autorisations TPV N° N° DATE DE ORIGINE BENEFICIAIRE AUTORISATIO CATEGORIE SERIE ITINERAIRE POINT DEPART POINT DESTINATION DOSSIER SEANCE CT D'AGREMENT N Casablanca - Beni Mellal et retour par Ben Ahmed - Kouribga - Oued Les Héritiers de feu FATHI Mohamed et FATHI Casablanca Beni Mellal 1 V 161 27/04/2006 Transaction 2 A Zem - Boujad Kasbah Tadla Rabia Boujad Casablanca Lundi : Boujaad - Casablanca 1- Oujda - Ahfir - Berkane - Saf Saf - Mellilia Mellilia 2- Oujda - Les Mines de Sidi Sidi Boubker 13 V Les Héritiers de feu MOUMEN Hadj Hmida 902 18/09/2003 Succession 2 A Oujda Boubker Saidia 3- Oujda La plage de Saidia Nador 4- Oujda - Nador 19 V MM. EL IDRISSI Omar et Driss 868 06/07/2005 Transaction 2 et 3 B Casablanca - Souks Casablanca 23 V M. EL HADAD Brahim Ben Mohamed 517 03/07/1974 Succession 2 et 3 A Safi - Souks Safi Mme. Khaddouj Bent Salah 2/24, SALEK Mina 26 V 8/24, et SALEK Jamal Eddine 2/24, EL 55 08/06/1983 Transaction 2 A Casablanca - Settat Casablanca Settat MOUTTAKI Bouchaib et Mustapha 12/24 29 V MM. Les Héritiers de feu EL KAICH Abdelkrim 173 16/02/1988 Succession 3 A Casablanca - Souks Casablanca Fès - Meknès Meknès - Mernissa Meknès - Ghafsai Aouicha Bent Mohamed - LAMBRABET née Fès 30 V 219 27/07/1995 Attribution 2 A Meknès - Sefrou Meknès LABBACI Fatiha et LABBACI Yamina Meknès Meknès - Taza Meknès - Tétouan Meknès - Oujda 31 V M. EL HILALI Abdelahak Ben Mohamed 136 19/09/1972 Attribution A Casablanca - Souks Casablanca 31 V M. -

Liste Des Guichets Des Banques Marocaines Par Localite Et Par Region
Programme Intégré d’Appui et de Financement des Entreprises LISTE DES GUICHETS DES BANQUES MAROCAINES PAR LOCALITE ET PAR REGION Février 2020 Programme Intégré d’Appui et de Financement des Entreprises LISTE DES GUICHETS DES BANQUES MAROCAINES PAR LOCALITE ET PAR REGION Février 2020 4 LISTE DES GUICHETS DES BANQUES MAROCAINES PAR LOCALITE ET PAR REGION TANGER – TÉTOUAN – AL HOCEIMA 5 L’ORIENTAL 13 FÈS - MEKNÈS 21 RABAT - SALÉ- KÉNITRA 29 BÉNI MELLAL- KHÉNIFRA 39 CASABLANCA- SETTAT 45 MARRAKECH - SAFI 65 DARÂA - TAFILALET 73 SOUSS - MASSA 77 GUELMIM - OUED NOUN 85 LAÂYOUNE - SAKIA EL HAMRA 87 DAKHLA-OUED EDDAHAB 89 LISTE DES GUICHETS DES BANQUES MAROCAINES PAR LOCALITE ET PAR REGION 5 TANGER – TÉTOUAN – AL HOCEIMA 6 RÉGION TANGER-TÉTOUAN-AL HOCEÏMA BANQUE LOCALITES GUICHET TELEPHONE AL BARID BANK AIT YOUSSEF OU ALI AIT YOUSSEF OU ALI CENTRE 0539802032 AJDIR CENTRE RURALE AJDIR 35052 TAZA 0535207082 AL AOUAMRA CENTRE AL AOUAMRA 92050 AL AOUAMRA 0539901881 AL HOCEIMA AVENUE MOULAY DRISS AL AKBAR AL HOCEIMA 0539982466 BV TARIK BNOU ZIAD AL HOCEIMA 0539982857 ARBAA TAOURIRT ARBAA TAOURIRT CENTRE 0539804716 ASILAH 1 PLACE DES NATIONS UNIES 90055 ASILAH 0539417314 ASMATEN CENTRE ASMATEN EN FACE EL KIADA AL HAMRA 93250 ASMATEN 0539707686 BAB BERRET CENTRE BAB BERRET 91100 BAB BERRET 0539892722 BAB TAZA CENTRE BAB TAZA 91002 BAB TAZA 0539896059 BENI BOUAYACHE BENI BOUAYACHE CENTRE 0539804020 BENI KARRICH FOUKI CENTRE BENI KARRICH FOUKI 93050 BENI KARRICH FOUKI 0539712787 BNI AHMED CENTRE BNI AHMED CHAMALIA 91100 BNI AHMED 0539881578 BNI AMMART -

B. Le Centre Hospitalier Provincial De Sidi Kacem
ﺳﺒﺤﺎﻧﻚ ﻻ ﻋﻠﻢ ﻟﻨﺎ ﺇﻻ ﻣﺎ ﻋﻠﻤﺘﻨﺎ ﺇﻧﻚ ﺃﻧﺖ ﺍﻟﻌﻠﻴﻢ ﺍﳊﻜﻴﻢ ﺳﻮﺭﺓ ﺍﻟﺒﻘﺮﺓ: ﺍﻵﻳﺔ: 31 UNIVERSITE MOHAMMED V FACULTE DE MEDECINE ET DE PHARMACIE RABAT DOYENS HONORAIRES : 1962 – 1969 : Professeur Abdelmalek FARAJ 1969 – 1974 : Professeur Abdellatif BERBICH 1974 – 1981 : Professeur Bachir LAZRAK 1981 – 1989 : Professeur Taieb CHKILI 1989 – 1997 : Professeur Mohamed Tahar ALAOUI 1997 – 2003 : Professeur Abdelmajid BELMAHI 2003 – 2013 : Professeur Najia HAJJAJ – HASSOUNI ADMINISTRATION : Doyen Professeur Mohamed ADNAOUI Vice Doyen chargé des Affaires Académiques et estudiantines Professeur Brahim LEKEHAL Vice Doyen chargé de la Recherche et de la Coopération Professeur Taoufiq DAKKA Vice Doyen chargé des Affaires Spécifiques à la Pharmacie Professeur Jamal TAOUFIK Secrétaire Général Mr. Mohamed KARRA 1- ENSEIGNANTS-CHERCHEURS MEDECINS ET PHARMACIENS PROFESSEURS : Décembre 1984 Pr. MAAOUNI Abdelaziz Médecine Interne – Clinique Royale Pr. MAAZOUZI Ahmed Wajdi Anesthésie -Réanimation Pr. SETTAF Abdellatif pathologie Chirurgicale Novembre et Décembre 1985 Pr. BENSAID Younes Pathologie Chirurgicale Janvier, Février et Décembre 1987 Pr. LACHKAR Hassan Médecine Interne Pr. YAHYAOUI Mohamed Neurologie Décembre 1989 Pr. ADNAOUI Mohamed Médecine Interne –Doyen de la FMPR Pr. OUAZZANI Taïbi Mohamed Réda Neurologie Janvier et Novembre 1990 Pr. HACHIM Mohammed* Médecine-Interne Pr. KHARBACH Aîcha Gynécologie -Obstétrique Pr. TAZI Saoud Anas Anesthésie Réanimation Février Avril Juillet et Décembre 1991 Pr. AZZOUZI Abderrahim Anesthésie Réanimation –Doyen de la FMPO Pr. BAYAHIA Rabéa Néphrologie Pr. BELKOUCHI Abdelkader Chirurgie Générale Pr. BENCHEKROUN Belabbes Abdellatif Chirurgie Générale Pr. BENSOUDA Yahia Pharmacie galénique Pr. BERRAHO Amina Ophtalmologie Pr. BEZZAD Rachid Gynécologie Obstétrique Méd Chef Maternité des Orangers Pr. CHERRAH Yahia Pharmacologie Pr. CHOKAIRI Omar Histologie Embryologie Pr. KHATTAB Mohamed Pédiatrie Pr. -

Pauvrete, Developpement Humain
ROYAUME DU MAROC HAUT COMMISSARIAT AU PLAN PAUVRETE, DEVELOPPEMENT HUMAIN ET DEVELOPPEMENT SOCIAL AU MAROC Données cartographiques et statistiques Septembre 2004 Remerciements La présente cartographie de la pauvreté, du développement humain et du développement social est le résultat d’un travail d’équipe. Elle a été élaborée par un groupe de spécialistes du Haut Commissariat au Plan (Observatoire des conditions de vie de la population), formé de Mme Ikira D . (Statisticienne) et MM. Douidich M. (Statisticien-économiste), Ezzrari J. (Economiste), Nekrache H. (Statisticien- démographe) et Soudi K. (Statisticien-démographe). Qu’ils en soient vivement remerciés. Mes remerciements vont aussi à MM. Benkasmi M. et Teto A. d’avoir participé aux travaux préparatoires de cette étude, et à Mr Peter Lanjouw, fondateur de la cartographie de la pauvreté, d’avoir été en contact permanent avec l’ensemble de ces spécialistes. SOMMAIRE Ahmed LAHLIMI ALAMI Haut Commissaire au Plan 2 SOMMAIRE Page Partie I : PRESENTATION GENERALE I. Approche de la pauvreté, de la vulnérabilité et de l’inégalité 1.1. Concepts et mesures 1.2. Indicateurs de la pauvreté et de la vulnérabilité au Maroc II. Objectifs et consistance des indices communaux de développement humain et de développement social 2.1. Objectifs 2.2. Consistance et mesure de l’indice communal de développement humain 2.3. Consistance et mesure de l’indice communal de développement social III. Cartographie de la pauvreté, du développement humain et du développement social IV. Niveaux et évolution de la pauvreté, du développement humain et du développement social 4.1. Niveaux et évolution de la pauvreté 4.2. -

Contribution À La Mesure De L'effet De L'innovation
Contribution à la mesure de l’effet de l’innovation sociale sur le développement territorial durable : Cas des projets sociaux dans la Région de Rabat-Salé-Kénitra, Maroc Driss El Ghoufi To cite this version: Driss El Ghoufi. Contribution à la mesure de l’effet de l’innovation sociale sur le développement territorial durable : Cas des projets sociaux dans la Région de Rabat-Salé-Kénitra, Maroc. Economies et finances. Université Cadi Ayyad de Marrakech (Maroc), 2019. Français. tel-02936971 HAL Id: tel-02936971 https://hal.archives-ouvertes.fr/tel-02936971 Submitted on 21 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE CADI AYYAD FACULTE DES SCIENCES JURIDIQUES, ECONOMIQUES ET SOCIALES MARRAKECH Centre des Etudes Doctorales : Droit, Economie et Gestion Laboratoire de recherche : Innovation, Responsabilité et Développement Durable (INREDD) THESE DE DOCTORAT EN : SCIENCES ECONOMIQUES CONTRIBUTION A LA MESURE DE L’EFFET DE L’INNOVATION SOCIALE SUR LE DEVELOPPEMENT TERRITORIAL DURABLE Cas des projets sociaux dans la Région de Rabat-Salé-Kénitra Par Driss EL GHOUFI Présentée et soutenue publiquement le 13/06/2019 Sous la direction du Professeur Fatima ARIB Jury : Mr. -
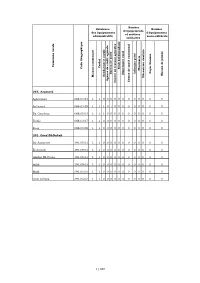
M a Is O N C O M M U N a Le C a Ïd a T G E N D a Rm E Rie Ro Y a Le A
Nombre Existence Nombre d'équipements des équipements d'équipements et services administratifs socio-culturels sanitaires Commune rurale Caïdat Code Géographique Souk Souk hebdomadaire Pharmacie Foyer féminin Infirmier privé Bureau deBureau poste Maison de jeunes Dispensaire rural Maison communale Mécanicien dentiste Gendarmerie royale Agence de crédit agricole Centre de santé communal Centre de travaux agricoles 066. Aousserd Aghouinite 066.03.03 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Aousserd 066.03.05 1 1 1 0 1 0 0 0 0 0 0 0 0 0 Bir Gandouz 066.05.03 1 1 1 0 0 0 0 0 0 0 0 0 0 0 Tichla 066.03.07 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Zoug 066.03.09 1 1 0 0 0 0 0 0 0 0 0 0 0 0 391. Oued Ed-Dahab Bir Anzarane 391.05.01 1 1 0 0 0 0 0 0 0 0 0 0 0 0 El Argoub 391.09.01 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Gleibat EL Foula 391.05.03 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Imlili 391.09.03 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Mijik 391.05.05 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Oum Dreyga 391.05.07 1 1 0 0 0 0 0 0 0 0 0 0 0 0 1/160 La commune Nombre d'établissements est Réseaux d'enseignement et de accessible d'infrastructure formation par Commune rurale Train Lycée Collège Autocar de développement ? Grand taxi Autre moyen Réseau d'électricité Réseau d'eau potable Ecole primaire satellite Ecole primaire centrale professionnelle publique Ecole coranique ou Msid Réseau d'assainissement La commune dispose-t-elle d'un plan Ecole primaire autonome Etablissement de formation 066. -

Télécharger Le Document
CARTOGRAPHIE DU DÉVELOPPEMENT LOCAL MULTIDIMENSIONNEL NIVEAU ET DÉFICITS www.ondh.ma SOMMAIRE Résumé 6 Présentation 7 1. Approche méthodologique 8 1.1. Portée et lecture de l’IDLM 8 1.2. Fiabilité de l’IDLM 9 2. Développement, niveaux et sources de déficit 10 2.1. Cartographie du développement régional 11 2.2. Cartographie du développement provincial 13 2.3. Développement communal, état de lieux et disparité 16 3. L’IDLM, un outil de ciblage des programmes sociaux 19 3.1 Causes du déficit en développement, l’éducation et le niveau de vie en tête 20 3.2. Profil des communes à développement local faible 24 Conclusion 26 Annexes 27 Annexe 1 : Fiabilité de l’indice de développement local multidimensionnel (IDLM) 29 Annexe 2 : Consistance et méthode de calcul de l’indice de développement local 30 multidimensionnel Annexe 3 : Cartographie des niveaux de développement local 35 Annexes Communal 38 Cartographie du développement communal-2014 41 5 RÉSUMÉ La résorption ciblée des déficits socio-économiques à l’échelle locale (province et commune) requiert, à l’instar de l’intégration et la cohésion des territoires, le recours à une cartographie du développement au sens multidimensionnel du terme, conjuguée à celle des causes structurelles de son éventuel retard. Cette étude livre à cet effet une cartographie communale du développement et de ses sources assimilées à l’éducation, la santé, le niveau de vie, l’activité économique, l’habitat et les services sociaux, à partir de la base de données «Indicateurs du RGPH 2014» (HCP, 2017). Cette cartographie du développement et de ses dimensions montre clairement que : - La pauvreté matérielle voire monétaire est certes associée au développement humain, mais elle ne permet pas, à elle seule, d’identifier les communes sous l’emprise d’autres facettes de pauvreté. -

Pris En Application Du Dahir Portant Loi N° 1-74-338 Du 24 Joumada II 1394 (15 Juillet 1974) Relatif À L’Organisation Judiciaire (B.O
Décret n° 2-74-498 (25 joumada II 1394) pris en application du dahir portant loi n° 1-74-338 du 24 joumada II 1394 (15 juillet 1974) relatif à l’organisation judiciaire (B.O. 17 juillet 1974) Décret n° 2-74-498 (25 joumada II 1394) pris en application du dahir portant loi n° 1-74-338 du 24 joumada II 1394 (15 juillet 1974) relatif à l’organisation judiciaire (B.O. 17 juillet 1974) Décret n° 2-74-498 (25 joumada II 1394) pris en application du dahir portant loi n° 1-74-338 du 24 joumada II 1394 (15 juillet 1974) relatif à l’organisation judiciaire Bulletin Officiel du 17 juillet 1974 Vu le dahir portant loi n° 1-74-338 du 24 joumada II 1394 (15 juillet 1974) fixant l’organisation judiciaire du Royaume ; Après examen par le Conseil des ministres réuni le 11 joumada II 1394 (2 juillet et 1974). Article Premier : L’organisation judiciaire comporte un certain nombre de juridictions dont le siège et le ressort sont fixés conformément au tableau annexé (1). Cours d’appel et tribunaux de première instance Tableau annexé au décret 2-74-498 tel qu’abrog. et rempl. par le n° 2-96-467 20 nov. 1996- 8 rejeb 1417 : BO 2 janv. 1997, p 3 tel que mod. par le décret 2-99-832 du 28 septembre 1999-17 joumada II 1420 ; par le Décret n° 2-00-732 du 2 novembre 2000 – 5 chaabane 1421- B.O du 16 novembre 2000 et par le décret n° 2-02-6 du 17 juillet 2002-6 joumada I 1423 (B.O du 15 août 2002, décret n° 2-03-884 du 4 mai 2004 – 14 rabii I 1425 ; B.O. -

Résultats Élections
REGION PREFECTURE CONSEIL COMMUNE NOM PRESIDENT ADRESSE OUED‐ED‐DAHAB‐ AOUSSERD commune LAGOUIRA brahim LAKHLIGUI CU. Lagouira, Hay Al MasjiD n° 41, Dakhla ‐ LAGOUIRA urbaine AousserD OUED‐ED‐DAHAB‐ OUED ED‐DAHAB ‐ commune DAKHLA (M) SiDi SLOH EL JAMANI CU. Dakhla ‐ OueD ED Dahab LAGOUIRA LAGOUIRA urbaine LAAYOUNE‐BOUJDOUR‐ LAAYOUNE commune EL MARSA (M) Hassan DERHEM CU. El Marsa, B.P. 36 ‐ Laâyoune SAKIA‐EL‐HAMRA urbaine LAAYOUNE‐BOUJDOUR‐ LAAYOUNE commune LAAYOUNE (M) HamDi OULED RACHID CU. Laâyoune, B.P. 495 ‐ Laâyoune SAKIA‐EL‐HAMRA urbaine LAAYOUNE‐BOUJDOUR‐ LAAYOUNE commune TARFAYA (M) AhmeD AZARQI CU. Tarfaya, B.P. 43 Tarfaya ‐ Laâyoune SAKIA‐EL‐HAMRA urbaine LAAYOUNE‐BOUJDOUR‐ BOUJDOUR commune BOUJDOUR (M) AbDelaziz ABBA CU. BoujDour, BD Hassan II ‐ BoujDour SAKIA‐EL‐HAMRA urbaine GUELMIM‐ES‐SEMARA TATA commune FAM EL HISN (M) MohameD OUDOR CU. Fam El Hisn – Tata urbaine GUELMIM‐ES‐SEMARA TATA commune TIZAGHTE Fatima BOUJNAH CR. Tizaghte, caïDat Issafen ‐ Tata rurale GUELMIM‐ES‐SEMARA TATA commune FOUM ZGUID (M) AbDerrahmane SAIDI CU. Foum ZguiD – Tata urbaine GUELMIM‐ES‐SEMARA TATA commune AKKA (M) RachiD MOULAY CHARIF CU. Akka – Tata urbaine GUELMIM‐ES‐SEMARA TAN TAN commune TAN TAN (M) Ali EL MAZLIOJ CU. Tan‐Tan – Tan‐Tan urbaine GUELMIM‐ES‐SEMARA ES SEMARA commune ES‐SEMARA (M) SiDi MohameD EL CU. Es‐Semara, Hôtel De ville, avenue urbaine JOUMANI MohameD V ‐ Es‐Semara GUELMIM‐ES‐SEMARA ASSA ZAG commune ZAG (M) Atman AILLA CU. Zag – Assa‐Zag urbaine GUELMIM‐ES‐SEMARA ASSA ZAG commune ASSA (M) HamDi OUAISSI CU. Assa – Assa‐Zag REGION PREFECTURE CONSEIL COMMUNE NOM PRESIDENT ADRESSE urbaine GUELMIM‐ES‐SEMARA GUELMIM commune GUELMIM (M) AbDlouhab BELFKIH CU. -

Annuaire Statistique Régional 2015
DIRECTION REGIONALE GHARB – CHRARDA – BENI HSSEN - 1 - - 2 - اـــــس ا اداري ..................................................................... ........................... .................................................. 9 ان .……………………………………………………………………………………………………… 17 اخ ............................................................................ ................ .................................................................. 27 ا و ا! ت وا ا#"ي ........ ..................................................................................................................... 33 ا%$ وا ء ............................. ................ ........................................................................................................ 51 ا(رة وا' ........................... ................ ...................................... .............................................................. 61 ا' ا+* ................ ................. ............................................................................................................... 71 ا -و, ت .......... ....................... ........................................................................................................................ 77 ا#ء وا-ر ...... ................. ............................................................................................................................. 85 ا. وا- .……………………………………………………………………………………………… 93 ا/ ...................... ................. ........................................................................................................................ -

Situation Du 31 Mars 2020
LISTE DES ETABLISSEMENTS ET ENTREPRISES AUTORISES : Situation du 31 Mars 2020 Numéro de Nom de Activité de Numéro d' Date de Direction régionale Service provincial Adresse Etat la ligne l'établissement l'établissement Autorisation délivrance Douar Abderrahim Ait El Toumecheghal, oued Entrepôts de stockage 1 SOUSS MASSA Agadir ES.27.456.19 11-mars-19 Autorisation délivrée Faqir Essafaa, Biougra, de produits alimentaires Chtouka Ait Baha N° 58 ZI, Sidi Bibi, 2 SOUSS MASSA Agadir AGRIFRESH Province chtouka Produits végétaux frais FLF.27.377.18 08-mars-18 Autorisation délivrée Ait Baha N° 3, Bloc B2, rue AGRO Issen, cité Alqods, Conservation de ALIMENTAIRE Agadir produits végétaux et 3 SOUSS MASSA Agadir TASSALTANTE CSTT.25.197.16 23-août-2016 Autorisation délivrée d’origine végétale sans ALQODS traitement thermique TANGER TETOUAN AL Z.I:imzouren P.d'al 4 Al Hoceima agrobiya Produits végétaux frais FLF.41.5.17 25-sept-17 Autorisation délivrée HOCEIMA hoceima Al Houda d'Huiles et Rue Miara QI Huiles issues de fruits 5 FES MEKNES FES HFO.09.143.19 27-mai-2019 Autorisation délivrée Céréales Doukkarat - Fès oléagineux 6 MARRAKECH SAFI MARRAKECH AS DISTRIBUTION N°424 Al Massar Produits végétaux frais FLF.19.127.16 14-juin-2016 Autorisation délivrée Stockage et conditionnement des 7 FES MEKNES Meknès Bab mansour Rue Essaadyine SCCL.13.58.16 22-juil-16 Autorisation délivrée céréales et légumineuses Entrepôts de stockage 8 FES MEKNES Meknès Bal developpement 69 bis Inbiaat 1 ESPA.13.57.16 30-mai-16 Autorisation délivrée de produits alimentaires 23, -

Liste Provisoire Des Revendeurs Des Pesticides À Usage Agricole Recensés
Liste provisoire des revendeurs des pesticides à usage agricole recensés Arrêté le 09 août 2019 Région Commune Ville / Localité Nom Adresse / Province Beni Mellal -khenifra Béni Mellal Ouled Mbarek AGRIMATCO SA Rue 20 aout Ouled Mbarek N°61 Béni Mellal Béni Mellal Comptoir Agricole du 51 Avenue des FAR Béni Mellal Souss Béni Mellal PROMAGRI Terrain 02 N°10 Zone industrielle Béni Mellal Promagri Lotissement 10 Quartier industrielle Béni Mellal Alphachimie Rue Hasssan 2, Ouled Hemdane, Dar Jdidada, Béni Mellal Béni Mellal SOCIETE KEMAGRO N 21 Zone industrielle , Béni Mellal Ouled Yaich STE Espace Horticole Douar Rquaba Ouled Yaich Béni Mellal Tadla SARL Ouled Mbarek Phyto Boutraba Rue 20 aout Ouled Mbarek Béni Mellal Béni Mellal Sté Filahati Elamriya 2, Hay El fath N°1 Béni Mellal Béni Mellal Sté Commerciale Siham Route de Marrakech KM3 Béni Mellal Aghbala STE AGRI-PHYTO QUARTIER ADMINSTRATIF AGHBALA AGHBALA SARL Fquih ben Souk Sebt Phyto Assimi 75 - 77 HAY NAJAT SOUK SEBT saleh Fquih ben RIAGRI SERVICE 128 AV DES FAR FQUIH BEN SALAH saleh Krifate ORA SEMENCES SARL DOUAR JDID COMMUENE KRIFAT FBS khénifra Aguelmous TASTAOUI YOUNESS RUE OULMASS AGUELMOUS khénifra MOSLIHI MOHAMED MARCH2 DAHAS PAM N° 10 KHENIFRA khénifra HAJJAJ BOUASSID KISARIA BEN DRISS N°1 KHENIFRA Mrirt BOUASSID KHALID 108 RUE ZAYTOUNE MRIRT MRIRT BOUASSID MUSTAPHA RUE ZAYTOUNE EN FACE DU MARCHE MRIRT khénifra BOUASSID MOHAMED 112, Boulvard la marche verte, Amalou khénifra bouhamidi el mostapha N° 3 IMMEUBLE DE HABOUSS Casablanca-Settat Benslimane Benslimane Ziaida Irrigation 93, Boulevard Beni Mekssal Bouznika Fellah Errachidia 43, Rue Khénifra, Bouzniza Berrechid Berrechid HYDROMECHAL N°55-57 RUE AL MASJID LOT AL YOUSSR - BERRECHID.