Cannabis Packaging and Labeling Regulatory Recommendations for States and Nations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FAQ About Recycling Cartons
FREQUENTLY ASKED QUESTIONS ABOUT CARTONS WHAT IS A CARTON? » Cartons are a type of packaging for food and beverage products you can purchase at the store. They are easy to recognize and are available in two types—shelf-stable and refrigerated. Shelf-stable cartons (types of products) Refrigerated (types of products) » Juice » Milk » Milk » Juice » Soy Milk » Cream » Soup and broth » Egg substitutes » Wine You will find these You will find these products in the chilled products on the shelves sections of grocery stores. in grocery stores. WHAT ARE CARTONS MADE FROM? » Cartons are mainly made from paper in the form of paperboard, as well as thin layers of polyethylene (plastic) and/or aluminum. Shelf-stable cartons contain on average 74% paper, 22% polyethylene and 4% aluminum. Refrigerated cartons contain about 80% paper and 20% polyethylene. ARE CARTONS RECYCLABLE? » Yes! Cartons are recyclable. In fact, the paper fiber contained in cartons is extremely valuable and useful to make new products. WHERE CAN I RECYCLE CARTONS? » To learn if your community accepts cartons for recycling, please visit RecycleCartons.com or check with your local recycling program. HOW DO I RECYCLE CARTONS? » Simply place the cartons in your recycle bin. If your recycling program collects materials as “single- stream,” you may place your cartons in your bin with all the other recyclables. If your recycling program collects materials as “dual-stream” (paper items together and plastic, metal and glass together), please place cartons with your plastic, metal and glass containers. WAIT, YOU JUST SAID CARTONS ARE MADE MAINLY FROM PAPER. Don’t I WANT TO PUT THEM WITH OTHER PAPER RECYCLABLES? » Good question. -

Different Perspectives for Assigning Weights to Determinants of Health
COUNTY HEALTH RANKINGS WORKING PAPER DIFFERENT PERSPECTIVES FOR ASSIGNING WEIGHTS TO DETERMINANTS OF HEALTH Bridget C. Booske Jessica K. Athens David A. Kindig Hyojun Park Patrick L. Remington FEBRUARY 2010 Table of Contents Summary .............................................................................................................................................................. 1 Historical Perspective ........................................................................................................................................ 2 Review of the Literature ................................................................................................................................... 4 Weighting Schemes Used by Other Rankings ............................................................................................... 5 Analytic Approach ............................................................................................................................................. 6 Pragmatic Approach .......................................................................................................................................... 8 References ........................................................................................................................................................... 9 Appendix 1: Weighting in Other Rankings .................................................................................................. 11 Appendix 2: Analysis of 2010 County Health Rankings Dataset ............................................................ -

Corrugated Board Structure: a Review M.C
ISSN: 2395-3594 IJAET International Journal of Application of Engineering and Technology Vol-2 No.-3 Corrugated Board Structure: A Review M.C. Kaushal1, V.K.Sirohiya2 and R.K.Rathore3 1 2 Assistant Prof. Mechanical Engineering Department, Gwalior Institute of Information Technology,Gwalior, Assistant Prof. Mechanical Engineering 3 Departments, Gwalior Engineering College, Gwalior, M. Tech students Maharanapratap College of Technology, Gwalior, [email protected] [email protected] [email protected] ABSTRACT Corrugated board is widely used in the packing industry. The main advantages are lightness, recyclability and low cost. This makes the material the best choice to produce containers devoted to the shipping of goods. Furthermore examples of structure design based on corrugated boards can be found in different fields. Structural analysis of paperboard components is a crucial topic in the design of containers. It is required to investigate their strength properties because they have to protect the goods contained from lateral crushing and compression loads due to stacking. However in this paper complete and detailed information are presented. Keywords: - corrugated boards, recyclability, compression loads. Smaller flutes offer printability advantages as well as I. INTRODUCTION structural advantages for retail packaging. Corrugated board is essentially a paper sandwich consisting of corrugated medium layered between inside II. HISTORY and outside linerboard. On the production side, corrugated In 1856 the first known corrugated material was patented is a sub-category of the paperboard industry, which is a for sweatband lining in top hats. During the following four sub-category of the paper industry, which is a sub-category decades other forms of corrugated material were used as of the forest products industry. -
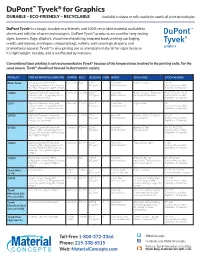
Tyvek Graphics Technical Data Sheet
DuPont ™ Tyvek ® for Graphics DURABLE – ECO-FRIENDLY – RECYCLABLE Available in sheets or rolls; usable for nearly all print technolo gies DuPont Tyvek a is a tough, durable, eco-friendly and 100% recyclable material available in sheets and rolls for all print technologies. DuPont Tyvek ® products are used for long-lasting signs, banners, flags, displays, visual merchandising, map and book printing, packaging, credit card sleeves, envelopes, shopping bags, wallets, wall coverings, drapery, and promotional apparel. Tyvek ® is also gaining use as a template material for signs because it is lightweight, durable, and is unaffected by moisture. Conventional laser printing is not recommended on Tyvek ® because of the temperatures involved in the printing units. For the same reason, Tyvek ® should not be used in electrostatic copiers. PRODUCT TYPE OF PRINTING USED FOR COATED MILS OZ. [GSM] CORE NOTES IDEAL USES STOCK WIDTHS Black Tyvek Flexography, Gravure, Offset Uncoated 5 mil 1.25 oz 2” Paper-like. Banners & Signs 36", 45" Lithography, Screen Process, UV-cure [42 gsm] Hard Structure Custom sizes available Inkjet (w/ testing due to lighter weight) Available in 10-yard rolls 1085D Digital on Demand, Flexography, Uncoated 10.3 mil 3.2 oz 3” Paper-like. Banners & Signs. Extra body 48.25", 57.125", 114.25" Gravure, Offset Lithography, Screen [109 gsm] Hard Structure for shape development. Custom sizes available Process, UV-cure Inkjet Available in 10-yard rolls 1079 Digital on Demand, Flexography, Uncoated 7.9 mil 2.85 oz 3” Paper-like. Tags & Labels 48" Gravure, Offset Lithography, Screen [97 gsm] Hard Structure Custom sizes available Process, Thermal Transfer, UV-cure Available in 10-yard rolls Inkjet 1073D Digital on Demand, Flexography, Uncoated 7.5 mil 2.2 oz 3” Paper-like. -

Getinge Pack Tyvek Sterilization Reels and Pouches
SAFETY DATA SHEET Getinge Pack Tyvek Sterilization Reels and Pouches 1.0 Product Product Name Getinge Pack Tyvek Sterilization Reels and Pouches and Company Product Code Tyvek Reel (TY)- Tyvek Pouch (TP) Identification Intended Use Tyvek Sterilization Reels and Pouches are intended to be used to enclose another medical devices that are to be sterilized by health care provider via related sterilization methods. Supplier PMS Tıbbi Cihazlar Teknolojisi Sanayi ve Ticaret A.Ş Identification Address Karaduvar Mah. Serbest Bölge 11. Cadde No: 46,Akdeniz, 33020 Mersin/TURKEY Phone +90 324 238 70 42 Fax +90 324 238 65 49 2.0 Hazards Tyvek material and PET/PE laminated film are not classified as dangerous according to EC directives and other international safety guidelines. Identification 3.0 Composition Information on Ingredients • Tyvek Material (1059B, 64.4 g/m² ) / Information on • Polyethylene Terephthalate / Polyethylene (PET/PE) Laminated Film Ingredients 12/50 microns • Printing inks, Indicator inks 4.0 First Aid Eye contact If molten material or dust contacts the eye, immediatly flush Measures with plenty of water for at least 15 minutes. If easy to do , remove contact lenses. Get medical attention immediately. Skin contact If contact with molten product occurs, treat as for thermal burn. Cool melted product on skin with plenty of water. Do not remove solidified product. If dust contacts the skin, immediately flush with plenty of water. Inhalation Remove patient to fresh air. Seek medical attention if necessary. Ingestion Material is not expected to be absorbed from the gastrointestinal tract. However, the printing chemicals such as printing inks and chemical indicator inks could be harmful. -

Label Liners: Meeting the Sustainability Challenge
Label liners: meeting the sustainability challenge By Mariya Nedelcheva, Product Manager Film & With ever-greater emphasis placed on packaging and waste reduction, Jenny Wassenaar, Compliance & Sustainability brand owners are looking for solutions that secure their sustainability Director, Avery Dennison credentials. Consumers pay particularly close attention to packaging, where there are significant environmental gains to be made. For example, waste from labelling can create useful by-products and circular economies. Waste is not always visible on the final packaging, but its impact on brand reputation is no less real. Consumers’ perceptions of a brand can be enhanced when sustainability is improved. label.averydennison.com Matrix Liner Final White paper waste waste label 16% 35% 37% Start-up waste plus Challenge printed End-user The challenge of recycling waste from the labelling process in error scrap - and ideally creating useful by-products - is complex. Many 10% 1% different elements must be addressed in order to move towards the ultimate goal of zero waste. For example, the word ‘recyclable’ can mean many things, and should not be viewed in isolation. Today there is a chance that recyclable products will still end up in landfill, so what matters is establishing genuinely viable end-to-end recycling solutions. That means considering every component within packaging, including where it comes from, how much material has been used, and what happens at every stage of the package’s journey through the value chain. This paper discusses how the sustainability of labelling laminates can be improved, with a particular focus on the label release liners that are left behind once labels have been dispensed. -

Coa Ng Conver Ng Distribu
s! t Last Tha ond A B Self-Adhesive Tapes Electrical Insula� on Tapes Industrial Tapes Packaging Tapes Double Sided Tapes Die-Cut Solu� ons Specialty Tapes Dispensers & Processing Devices Coa� ng Conver� ng Distribu� on Volz Selbstklebetechnik GmbH YOUR EXPERT IN ADHESIVE TAPE SOLUTIONS VOLZ® TAPES is your competent partner for all self-adhesive tape requirements. Our extensive product por� olio offers a variety of standard and specialty tape products, suppor� ng advanced industry applica� ons, as well as standard and complex packaging require- ments. We are the industry expert in double-sided tapes, electrical and industrial tapes, packaging tapes, self-adhesive die-cut solu� ons, as well as tape dispensers and processing devices. VOLZ® TAPES has spent the last several decades developing its core competencies in the areas of Coa� ng, Conver� ng and Distribu� on. We produce turn-key custom tape solu� ons to meet our customers’ most complex industry challenges. Our advanced produc� on facility is capable of cu� ng any tape width or producing die-cut solu� ons to your exact specifica� ons. It is our goal to develop high-end, cost effec� ve tape solu� ons that meet your opera� onal demands. VOLZ® TAPES is an ac� ve and trusted member of AFERA, the European Adhesive Tape Associa� on. AFERA membership provides a front row seat to market developments and valuable exposure to other key adhesive tape associa� ons such as PSTC (USA), CATIA (China), TAAT (Taiwan) and JATMA (Japan). • Coa� ng of diverse carrier materials such as Fabric, PP, PE, PET, PVC, Fleece and COATING: CUSTOMIZED ADHESIVE SOLUTIONS Foam VOLZ® TAPES develops and manufactures high-end electrical and • Available Adhesives Include: double-sided adhesive tapes. -

U.S. Postal Service Mail Addressing Guidelines
U.S. Postal Service Mail Addressing Guidelines Address Placement Placement of the address on the face of an envelope should conform to the following U. S. Postal Service specifications. • The address should be in an area, one inch from each side of the envelope. • The top of the address should be no more than 2 3/4 inches from the bottom of the envelope and the bottom no more than 5/8's of an inch from the bottom of the envelope. • The area 4 1/2 inches by 5/8 's of an inch in the lower right hand corner of the envelope MUST remain empty for bar code placement for any maul that is processed by the Mail Center. Enclosures Correspondence Mail of any kind for transport by the U. S. Postal Service must be enclosed in an appropriate envelope or parcel and sealed. The type of enclosures determine the mailing classification. Non-mailables Several items are listed by the U. S. Postal Service as non-mailable in envelopes: • paper clips • metal pieces • glass, chips • sand. These can jam or damage the mailing machines and can cause serious injury to Mail Center and Postal employees. The following are also classified as non-mailables and subject to return to sender: envelopes and cards less than 3 1/2 inches in height or 5 inches in length. It is recommended that when mailing questionable items to U. S. or foreign destinations, the mailer should contact the Mail Center for assistance. All foreign countries also impose various restrictions. Brochures, letters and newsletters being mailed without an envelope must be folded consistently and must be tabbed with the recommended number of tabs. -

The HP Molded Fiber Advanced Tooling Solution White Paper | the HP Molded Fiber Advanced Tooling Solution
White paper The HP Molded Fiber Advanced Tooling Solution White paper | The HP Molded Fiber Advanced Tooling Solution Macrotrends driving the packaging market The packaging market is transitioning from foam/plastic to molded fiber (also known as molded pulp) packaging for sustainability reasons, with environmental packaging (i.e., replacing foam with paper) favoring the growth of this segment. The molded fiber packaging market is a worldwide industry, valued at $8 billion, and is the fastest-growing segment in the packaging market, with growth being fueled by growing consumer demand for sustainable and environmentally friendly packaging solutions, as well as the increase in take-out food and e-commerce.1 Asia-Pacific countries (APAC) are the biggest geographical market, especially China, Hong Kong, and Taiwan. The region is experiencing the highest growth and is expected to be the largest market by 2024. This growth can be attributed to the rising importance of disposable packaging,2 as well as the lower cost of tooling production in these areas, which presents a significant price advantage over other regions. The primary segments currently are food and beverage and electronics, which are estimated to have the highest growth. The increasing use of molded pulp products for the packaging of various electronic products (including mobile phones and accessories, computers, printers, modems, trimmers, and projectors, among others) due to its convenience, improved sustainability, and low cost are some of the factors contributing to the growth of this segment.2 Molded fiber manufacturing The thermoformed tooling segment is expected to outgrow other segments, propelled by increased consumer demand for high-quality molded fiber packaging and ongoing innovation driving down thermoforming-packaging manufacturing costs. -

Dupont™ Tyvek® Water-Resistive Barriers Installation Guidelines
DuPont™ Tyvek® Water-Resistive Barriers Installation Guidelines HELPING YOU GET THE JOB DONE RIGHT VERSION 2 Table of Contents Applicable Products ..................................................................................................................................................................2 Recommended Materials .........................................................................................................................................................2 Code Requirements ..................................................................................................................................................................3 General Instructions .................................................................................................................................................................3 Special Considerations .............................................................................................................................................................3 Installation Instructions .............................................................................................................................................................4 Continuity Terminations ........................................................................................................................................................................6 Gable Ends ...........................................................................................................................................................................6 -

Supply Chain Packaging Guide
Secondary Packaging Supply Chain Standards July 7, 2021 Business Confidential | ©2021 Walmart Stores, Inc. 177 // 338 Secondary Packaging Supply Chain Standards - Update Summary These standards have included multiple clarifications of what is required and what is NOT ALLOWED. These changes have been updated throughout the published standards to provide clarity to suppliers. The pages have been reorganized to provide a better flow. PAGE 2021 UPDATES Changes to Supply Chain Standards 185 SQEP Phase 2 and Phase 3 Defect Description/Definitions Added 202 General Case Markings Updated for Dates, Unprocessed Meats, and Cylindrical Items 210-213 Updated Pallet Standards 218 Update "Palletized Shipments" to "Unitized Shipments" 227 Add Inbound Appointment Scheduling Standard 228 Update TV Test Standards 235-237 Add Direct Store Delivery (DSD) aka Direct To Store (DTS) Standards 239 Update SIOC Standards 240 Add eCommerce Product Specific Requirement Standards 241-244 Add Drop Ship Vendor (DSV) Standards 268 Add Jewelry Distribution Center Standards 269-271 Add Optical Distribution Center Standards 275 Add Goods Not For Resale (GNFR) Standards 277-278 Update Meat/Poultry/Seafood Case and Pallet Label Standards 284 Add HACCP Pallet Placard for GCC Shipments 311-312 Add Frozen Seafood Carton Marking Requirements Appendix D Update Receiving Pulp Temperature Range Business Confidential | © 2021 Walmart Stores, Inc. The examples shown are for reference only. Supply Chain Standards 178 // 338 Table of Contents Supply Chain Stretch Wrap . 219 Produce Shipments . 280 Contact Information . 179 Trailer Loading . 220 Automated Grocery Handling . 281 Walmart Retail Link Resources . 180 Trailer Measurements. 221 Grocery Import Distribution Center (GIDC) . 282 Walmart Distribution Center Overview . -

Pfass and Alternatives in Food Packaging (Paper and Paperboard): Report on the Commercial Availability and Current Uses
PFASs and alternatives in food packaging (paper and paperboard): Report on the commercial availability and current uses Series on Risk Management No. 58 1 Series on Risk Management 0 No. 58 PFASs and Alternatives in Food Packaging (Paper and Paperboard) Report on the Commercial Availability and Current Uses PUBE Please cite this publication as: OECD (2020), PFASs and Alternatives in Food Packaging (Paper and Paperboard) Report on the Commercial Availability and Current Uses, OECD Series on Risk Management, No. 58, Environment, Health and Safety, Environment Directorate, OECD. Acknowledgements: The OECD would like to acknowledge the drafting of a consultancy report by Steve Hollins of Exponent International Ltd. upon which this report is based. It was prepared under the framework of the OECD/UNEP Global PFC Group and included the contribution of information by several organisations (see Annex A). The report is published under the responsibility of the OECD Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. © Photo credits: Cover: Yuriy Golub/Shutterstock.com © OECD 2020 Applications for permission to reproduce or translate all or part of this material should be made to: Head of Publications Service, [email protected], OECD, 2 rue André-Pascal, 75775 Paris Cedex 16, France ABOUT THE OECD 3 About the OECD The Organisation for Economic Co-operation and Development (OECD) is an intergovernmental organisation in which representatives of 36 industrialised countries in North and South America, Europe and the Asia and Pacific region, as well as the European Commission, meet to co-ordinate and harmonise policies, discuss issues of mutual concern, and work together to respond to international problems.