S Angola on Their Way South
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Angola 3727 R4 HR
K Brazzaville asa CONGO i Kinshasa ANGOLA A D IN re B i Kikwit A a DEMOCRATIC C Z K K w REPUBLIC w i Cabinda a l OF THE u n g e o CONGO ng Nóqui oa K L Soyo M'banza Congo w e ZAIREZAIRE e Quimbele n ANGOLA dg Damba C W g K ri e b u a a M s a m a UÍGEUÍGE n L i Bembe g b o a u N'zeto Bungo a n e g Dundo og u L Uíge e a Negage p Ambriz a U Cuilo c Andrada i a de Marimba m h an Camabatela C Caxito D b Lucapa L LUNDA o LUNDA u KUANZAKUANZA Luremo a c b s O a s Luanda m NORTE NORTE l NORTE O NORTE a a a Cuango L G N'dalatando C C u G Quela Lubalo l LUANDA N u N Malanje o a Lucala l E Dondo i E u Saurimo Cabo Ledo uanza Cangandala Xá-Muteba B C C M DEMOCRATIC Cabo de São Bráz ALA LUNDALUNDA L KUANZAKUANZA L N REPUBLIC on ua J Cacolo SUL ga n E SUL OF THE Mussende do Quibala Quirima Muconda Porto Amboim SUL u CONGO SUL o a t Lu Gabela a ATLANTIC t Quimbango sai Cuvo ou Uaco u lo s Q du a Sumbe C n C u Cungo A vo OCEAN u Bimbe Nharea Lumeje Cassongue e Camacupa Luena ez HUAMBO Chicala b Cazombo Balombo Luatamba m Lobito Cuemba a Lucusse Z Benguela BENGUELA Huambo Kuito L o ung ng Lumbala Ponta das Salinas ué-Bu C Cubal op or olo Ganda Cuima Sambo BIÉ L Cabo de MOXICO un Santa Maria gw Zambezi C e bu Chitembo C u n Cabo de Caconda u a g i n u Santa Marta t Lumbala Quilengues o Q d u o N'guimbo Cubango e NAMIBE Menongue m HUÍLAHUÍLA Cuchi b o Bibala Matala C u b Lubango Techamutete a Chiume e n Chibia n g Cuíto ZAMBIA Namibe e o n Cuanavale u Virei Chiange C Cuvelai Mavinga Tombua KUANDO-KUBANGO U Curoc KUANDO-KUBANGO te a CUNENECUNENE m Cahama bo Savate Z Xangongo C am u b n i e ne Ondjiva to z u i C Chitado Santa Clara Cuangar Luiana Cubango NAMIBIA Mucusso National capital International boundary Provincial capital Provincial boundary BOTSWANA Town, village Road 0 50 100 150 200 km The boundaries and names shown and the Airport Track designations used on this map do not imply official endorsement or acceptance by the Railroad 0 50 100 150 mi United Nations. -

Project Brown Field Ambriz Yard
CSR Field Trip – Angola, November 2014 PROJECT BROWN FIELD AMBRIZ YARD ZONE D’IMAGE Denis Pascal PBF HSE Manager CSR Field Trip – Angola, November 2014 1 “Projectos Brown Field” in a nutshell An innovative project organization • PBF team set up in 2012 within Total E&P Angola • Objective to increase recovery rates on Block 17 – Optimizing existing installations – Developing satellite fields • Enhancing synergies and consistency • In charge of Girassol Resources Initiative (GirRI), Dalia Infills, Dalia Debottlenecking, Pazflor Infills and Zinia Phase 2 An approach prioritizing local content • Team based in Angola from start-up to ensure skills and knowledge transfer • Local companies used for basic engineering studies – More than 100,000 hours commissioned • Local fabrication by Petromar yard in Ambriz Maximizing production through high-tech local content CSR Field Trip – Angola, November 2014 2 Block 17 PBF, a high technology project GirRI Rosa MPP Dalia phase 1A Acacia infills 42 Mb 52 Mb 17 Mb 20 kb/d plateau 24 kb/d plateau 12 kb/d peak • 2 high boost multiphase • 3 producers and 1 injector pump modules • 7 producer well systems well systems • Multiphase pump module • 3 flowbases • 2 satellite manifolds integration on Girassol • Dalia FPSO subsea control • Configuration of Pazflor • Power interconnection system upgrade FPSO control system between Girassol and Dalia CSR Field Trip – Angola, November 2014 3 GirRi Rosa MPP, a show-case for local content Local content in GirRI Rosa MPP project Inside contracts, end of project forecast Local -

Download/Pdf/132634899.Pdf
THE END OF CATTLE’S PARADISE HOW LAND DIVERSION FOR RANCHES ERODED FOOD SECURITY IN THE GAMBOS, ANGOLA Amnesty International is a global movement of more than 9 million people who campaign for a world where human rights are enjoyed by all. Our vision is for every person to enjoy all the rights enshrined in the Universal Declaration of Human Rights and other international human rights standards. We are independent of any government, political ideology, economic interest or religion and are funded mainly by our membership and public donations. © Amnesty International 2019 Except where otherwise noted, content in this document is licensed under a Creative Commons Cover photo: Girl leading a pair of oxen pulling a traditional cart in the Gambos, (attribution, non-commercial, no derivatives, international 4.0) licence. Angola © Amnesty International https://creativecommons.org/licenses/by-nc-nd/4.0/legalcode For more information please visit the permissions page on our website: www.amnesty.org Where material is attributed to a copyright owner other than Amnesty International this material is not subject to the Creative Commons licence. First published in 2019 by Amnesty International Ltd Peter Benenson House, 1 Easton Street London WC1X 0DW, UK Index: AFR 12/1020/2019 Original language: English amnesty.org CONTENTS GLOSSARY 5 ACKNOWLEDGEMENTS 7 EXECUTIVE SUMMARY 8 METHODOLOGY 14 THE GAMBOS 16 FOOD INSECURITY IN THE GAMBOS 19 DECLINING MILK PRODUCTION 19 DECLINING FOOD PRODUCTION 23 HUNGER AND MALNUTRITION 24 THE ROOT OF THE PROBLEM 26 LAND DISPOSSESSION AND FOOD SECURITY 27 CATTLE ARE OUR LIFE 29 THE SPECIAL STATUS OF TUNDA AND CHIMBOLELA 31 ECONOMIC VALUES OF CATTLE 32 “THE CATTLE ARE OUR BANK, INSURANCE AND SOCIAL SECURITY” 32 “THE CATTLE GIVE US EDUCATION” 33 “THE CATTLE ARE OUR TRACTORS” 34 FAILURE TO PREVENT LAND DISPOSSESSION 37 EVIDENCE FROM SATELLITE 38 EVIDENCE FROM THE GOVERNMENT 38 EVIDENCE FROM THE PASTORALISTS 40 1. -

Further Breeding Records for Birds (Aves) in Angola
Durban Natural Science Museum Novitates 36 ANGOLAN BIRD BREEDING RECORDS 1 FURTHER BREEDING RECORDS FOR BIRDS (AVES) IN ANGOLA W. RicHARD J. DeAn1*, URSULA FRAnKe2, GRAnT JOSePH1, FRANCIScO M. GOnÇALVeS3, MicHAeL S.L. MiLLS4,1, SUZAnne J. MiLTOn1, ARA MOnADJeM5 & H. DieTeR OScHADLeUS6 1DST/NRF Centre of Excellence at the Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, South Africa *Author for correspondence: [email protected] 2Tal 34, 80331 Munich, Germany 3ISCED, Department of Natural Sciences, Rua: Sarmento Rodrigues, P.O. Box 230, Lubango, Angola 4A.P. Leventis Ornithological Research Institute, University of Jos, P.O. Box 13404, Jos, Plateau State, Nigeria 5Department of Biological Sciences, University of Swaziland, Private Bag 4, Kwaluseni, Swaziland 6Animal Demography Unit, Department of Zoology, University of Cape Town, Rondebosch 7701, South Africa ean, W.R.J., Franke, U., Joseph, G., Gonçalves, F.M., Mills, M.S.L., Milton, S.J., Monadjem, A. D& Oschadleus, H.D. 2013. Further breeding records for birds (Aves) in Angola. Durban Natural Science Museum Novitates 36: 1-10. Some details of records of nests, eggs and nestlings of 167 (possibly 168) species in the bird collection at Lubango, Angola are given. This includes 23 species for which there were no Angolan breeding records at all, and one possibly new breeding species (Slaty Egret). The data also confirm the breeding of another 20 species strongly suspected of breeding in Angola, but that lacked egg or nestling records. KEYWORDS: Angola, birds, museum collections, breeding. INTRODUcTiOn SYSTeMATIC LiST One of the gaps in our knowledge of the natural history of birds in Taxonomy and order follows Gill & Donsker (2014). -
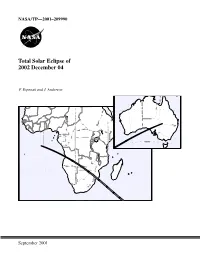
Total Solar Eclipse of 2002 December 4
NASA/TP—2001–209990 Total Solar Eclipse of 2002 December 04 F. Espenak and J. Anderson Central Lat,Lng = -28.0 132.0 P Factor = 0.46 Semi W,H = 0.35 0.28 Offset X,Y = 0.00-0.00 1999 Oct 26 10:40:42 AM High Res World Data [WPD1] WorldMap v2.00, F. Espenak Orthographic Projection Scale = 8.00 mm/° = 1:13915000 Central Lat,Lng = -10.0 26.0 P Factor = 0.31 Semi W,H = 0.70 0.50 Offset X,Y = 0.00-0.00 1999 Oct 26 10:17:57 AM September 2001 The NASA STI Program Office … in Profile Since its founding, NASA has been dedicated to • CONFERENCE PUBLICATION. Collected the advancement of aeronautics and space papers from scientific and technical science. The NASA Scientific and Technical conferences, symposia, seminars, or other Information (STI) Program Office plays a key meetings sponsored or cosponsored by NASA. part in helping NASA maintain this important role. • SPECIAL PUBLICATION. Scientific, techni- cal, or historical information from NASA The NASA STI Program Office is operated by programs, projects, and mission, often con- Langley Research Center, the lead center for cerned with subjects having substantial public NASA’s scientific and technical information. The interest. NASA STI Program Office provides access to the NASA STI Database, the largest collection of • TECHNICAL TRANSLATION. aeronautical and space science STI in the world. English-language translations of foreign scien- The Program Office is also NASA’s institutional tific and technical material pertinent to NASA’s mechanism for disseminating the results of its mission. -

Africa Notes
Number 137 June 1992 CSISAFRICA NOTES A publication of the Center for Strategic and International Studies , Washington, D.C. Angola in Transition: The Cabinda Factor by Shawn McCormick In accordance with the Portuguese-mediated agreement signed by leaders of the governing Movimento Popular de Libertac;:ao de Angola (MPLA) .and the Uniao Nacional para a Independencia Total de Angola (UNIT A) in May 1991, the 16-year civil war that erupted in Angola as the country achieved independent statehood in 1975 has ended. Efforts to implement the second priority mandated in the agreement-national elections by late 1992-are being assisted by a range of international actors, including the United Nations, the United States, Russia, and Portugal. More than 12 parties are likely to participate in the elections (scheduled for September 29 and 30, 1992). The process of achieving a third key element of the agreement-demobilization of three-fourths of the two armies and integration of the remaining soldiers into a 50,000-strong national force-seems unlikely to conclude before elections are held. Although media attention focuses on developments and major players in the capital city of Luanda, where UNIT A has officially established a presence, analysts of the Angolan scene are according new attention to tiny Cabinda province (where an increasingly active separatist movement is escalating its pursuit of independence from Luanda) as "possibly Angola's last and most important battlefield." The significance of Cabinda-a 2,807-square-mile enclave along the Atlantic Ocean separated from Angola's other 17 contiguous provinces by a 25-mile strip of Zaire-lies in the fact that current offshore oil production, including that from the Takula and Malanga fields, totals more than 310,000 barrels per day (bpd). -

Kimberlites Associated with the Lucapa Structure, Angola
Kimberlites associated with the Lucapa structure, Angola Sandra Elvira Robles Cruz ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde un sitio ajeno al servicio TDR. No se autoriza la presentación de su contenido en una ventana o marco ajeno a TDR (framing). Esta reserva de derechos afecta tanto al resumen de presentación de la tesis como a sus contenidos. En la utilización o cita de partes de la tesis es obligado indicar el nombre de la persona autora. -

Órgão Oficial Da República De Angola
Sexta-feira, 29 de Junho de 2018 I Série – N.º 94 DIÁRIO DA REPÚBLICA ÓRGÃO OFICIAL DA REPÚBLICA DE ANGOLA Preço deste número - Kz: 1.780,00 !"#$% $% &"''()*"+#+&-$.% /0('% "[&-$2.% /0('% ASSINATURA [%*'( "%#(%&$#$%2-+H$%*0:2-&$#$%+")%7-'-")% '(2$3-4$% $% $++&-"% (% $))-+$30'$)% #"% E7-'-"% Ano #$%9(*:2-&$%MC9%(%JC9%) '-(% %#(%TUR%VXCOO%(%*$'$% #$% 9(*:2-&$F.% #(4(% )('% #-'-<-#$% % >?*'(+)$% K)%3')%) '-()% %CCCC CCC CCC CCC CCC CCC% TUR%PMM%VWWCXO $% NC9% ) '-(% TUR% WXCOO.% $&'()&-#"% #"% '()*(&3-4"% @$&-"+$2% A% BCDC.% (?% E0$+#$.% 90$% F(+'-/0(% #(% K%MC9%) '-(% %CCCC CCC CCC CCC CCC CCC% TUR%NPM%JVOCOO -?*")3"%#"%)(2".%#(*(+#(+#"%$%*0:2-&$ "%#$% G$'4$2H"% +C:% J.% G-#$#(%K23$.% G$-L$% D")3$2% MNOP.% QQQC-?*'(+)$+$&-"+$2C<"4C$"% A% B+#C% 3(2(<CR% K%JC9%) '-(% %CCCC CCC CCC CCC CCC CCC% TUR%MZW%MXOCOO NC9%) '-(%#(%#(*)-3"%*' 4-"%$%(_(&30$'%+$%3()"0A E>?*'(+)$FC K%NC9%) '-(% %CCCC CCC CCC CCC CCC CCC% TUR%MXO%MMMCOO '$'-$%#$%>?*'(+)$%@$&-"+$2%A%BC%DC SUMÁRIO ARTIGO 1.º (Aprovação) É aprovado o Plano de Desenvolvimento Nacional 2018- Presidente da República BCBB1'&$",*'&*'(3"4"$/"'D"-3"/*'53"4%!"$-%&)'"'!")"'(&3/"' Decreto Presidencial n.º 158/18: integrante. Aprova o Plano de Desenvolvimento Nacional 2018-2022. ARTIGO 2.º Decreto Presidencial n.º 159/18: (Dúvidas e omissões) Nomeia Elisa Rangel Nunes para o cargo de Juíza Conselheira do Tribunal de Contas, Joaquim Mande para o cargo de Juiz Conselheiro do Tribunal As dúvidas e omissões resultantes da interpretação e apli- de Contas e Rigoberto Kambovo para o cargo de Juiz Conselheiro cação do presente Diploma são resolvidas pelo Presidente da do Tribunal de Contas. República. ARTIGO 3.º PRESIDENTE DA REPÚBLICA (Entrada em vigor) O presente Decreto Presidencial entra em vigor na data da sua publicação. -

2.3 Angola Road Network
2.3 Angola Road Network Distance Matrix Travel Time Matrix Road Security Weighbridges and Axle Load Limits For more information on government contact details, please see the following link: 4.1 Government Contact List. Page 1 Page 2 Distance Matrix Uige – River Nzadi bridge 18 m-long and 4 m-wide near the locality of Kitela, north of Songo municipality destroyed during civil war and currently under rehabilitation (news 7/10/2016). Road Details Luanda The Government/MPLA is committed to build 1,100 km of roads in addition to 2,834 km of roads built in 2016 and planned rehabilitation of 7,083 km of roads in addition to 10,219 km rehabilitated in 2016. The Government goals will have also the support from the credit line of the R. of China which will benefit inter-municipality links in Luanda, Uige, Malanje, Cuanza Norte, Cuanza Sul, Benguela, Huambo and Bié provinces. For more information please vitsit the Website of the Ministry of Construction. Zaire Luvo bridge reopened to trucks as of 15/11/2017, this bridge links the municipality of Mbanza Congo with RDC and was closed for 30 days after rehabilitation. Three of the 60 km between MCongo/Luvo require repairs as of 17/11/2017. For more information please visit the Website of Agencia Angola Press. Works of rehabilitation on the road nr, 120 between Mbanza Congo (province Zaire) and the locality of Lukunga (province of Uige) of a distance of 111 km are 60% completed as of 29/9/2017. For more information please visit the Website of Agencia Angola Press. -

Final Report: Southern Africa Regional Environmental Program
SOUTHERN AFRICA REGIONAL ENVIRONMENTAL PROGRAM FINAL REPORT DISCLAIMER The authors’ views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States government. FINAL REPORT SOUTHERN AFRICA REGIONAL ENVIRONMENTAL PROGRAM Contract No. 674-C-00-10-00030-00 Cover illustration and all one-page illustrations: Credit: Fernando Hugo Fernandes DISCLAIMER The authors’ views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States government. CONTENTS Acronyms ................................................................................................................ ii Executive Summary ............................................................................................... 1 Project Context ...................................................................................................... 4 Strategic Approach and Program Management .............................................. 10 Strategic Thrust of the Program ...............................................................................................10 Project Implementation and Key Partners .............................................................................12 Major Program Elements: SAREP Highlights and Achievements .................. 14 Summary of Key Technical Results and Achievements .......................................................14 Improving the Cooperative Management of the River -

Proyecto De Arquitectura
AGENDA DE ACÇÃO DE ANGOLA SUSTAINABLE ENERGY FOR ALL – ACTION AGENDA - ANGOLA Se4All. Agenda Acçao. Angola Se4All. Agenda Acçao. Angola Se4All. Agenda Acçao. Angola ÍNDICE Prefácio ....................................................................................................................................................... 11 RESUMO EXECUTIVO (Português). ............................................................................................................. 14 EXECUTIVE SUMMARY (English) ................................................................................................................. 20 1. INTRODUÇÃO. ........................................................................................................................................ 25 1.1. A iniciativa SE4ALL. .......................................................................................................................... 25 1.2. SE4All em Angola. ............................................................................................................................ 31 2. SITUAÇÃO EM ANGOLA. ......................................................................................................................... 33 2.1. Situação Geral do País. .................................................................................................................... 33 2.2. Sector energético Angolano. ........................................................................................................... 38 2.3. Planos de Desenvolvimento doSector -

Acdsee Print
COMO PODEM AS COMUNIDADES COSTEIRAS ENVOLVER-SE E BENEFICIAR DO PROGRAMA BCLME : UMA ANÁLISE I. RELATÓRIO DA VISITA A ANGOLA Fevereiro 2004 Como Podem as Comunidades Costeiras Envolver-se e Beneficiar do Programa BCLME: Uma Análise I. Relatório da Visita a Angola i O Programa do Grande Ecossistema Marinho da Corrente de Benguela (BCLME) visa a gestão deste ecossistema único de afloramento costeiro que acompanha as costas de Angola, Namíbia e África do Sul. Financiado pelo portfólio de Águas Internacionais do Fundo para o Ambiente Mundial (GEF), o Programa é implementado pelo Programa das Nações Unidas para o Desenvolvimento (PNUD), sendo o Gabinete das Nações Unidas para os Serviços de Apoio aos Projectos (UNOPS) a agência executora. Os três países membros asseguram financiamento adicional através de contribuições em espécie. Ao longo dos 4 000 km de costa vivem comunidades que dependem, a diferentes níveis, dos recursos naturais deste ecossistema, desempenhando um papel importante na gestão e saúde dos recursos costeiros. Ainda que o envolvimento das comunidades costeiras não seja o foco principal do Programa, é cada vez mais aceite que as actividades ao nível comunitário podem contribuir significativamente para o sucesso global do Programa, ao mesmo tempo que criam oportunidades para desenvolvimento comunitário. Foi neste contexto que a EcoAfrica Environmental Consultants realizou um estudo de ‘primeira paroximação’ para analisar como as comunidades costeiras podem contribuir para a gestão do BCLME e posicionar-se de modo a obter o máximo proveito dos recursos costeiros, bem como para recomendar qual o papel que o Programa BCLME pode desempenhar para atingir este objectivo.