The Root Systems of Onion and Allium Fistulosum in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

Full of Beans: a Study on the Alignment of Two Flowering Plants Classification Systems
Full of beans: a study on the alignment of two flowering plants classification systems Yi-Yun Cheng and Bertram Ludäscher School of Information Sciences, University of Illinois at Urbana-Champaign, USA {yiyunyc2,ludaesch}@illinois.edu Abstract. Advancements in technologies such as DNA analysis have given rise to new ways in organizing organisms in biodiversity classification systems. In this paper, we examine the feasibility of aligning two classification systems for flowering plants using a logic-based, Region Connection Calculus (RCC-5) ap- proach. The older “Cronquist system” (1981) classifies plants using their mor- phological features, while the more recent Angiosperm Phylogeny Group IV (APG IV) (2016) system classifies based on many new methods including ge- nome-level analysis. In our approach, we align pairwise concepts X and Y from two taxonomies using five basic set relations: congruence (X=Y), inclusion (X>Y), inverse inclusion (X<Y), overlap (X><Y), and disjointness (X!Y). With some of the RCC-5 relationships among the Fabaceae family (beans family) and the Sapindaceae family (maple family) uncertain, we anticipate that the merging of the two classification systems will lead to numerous merged solutions, so- called possible worlds. Our research demonstrates how logic-based alignment with ambiguities can lead to multiple merged solutions, which would not have been feasible when aligning taxonomies, classifications, or other knowledge or- ganization systems (KOS) manually. We believe that this work can introduce a novel approach for aligning KOS, where merged possible worlds can serve as a minimum viable product for engaging domain experts in the loop. Keywords: taxonomy alignment, KOS alignment, interoperability 1 Introduction With the advent of large-scale technologies and datasets, it has become increasingly difficult to organize information using a stable unitary classification scheme over time. -

Generic Classification of Amaryllidaceae Tribe Hippeastreae Nicolás García,1 Alan W
TAXON 2019 García & al. • Genera of Hippeastreae SYSTEMATICS AND PHYLOGENY Generic classification of Amaryllidaceae tribe Hippeastreae Nicolás García,1 Alan W. Meerow,2 Silvia Arroyo-Leuenberger,3 Renata S. Oliveira,4 Julie H. Dutilh,4 Pamela S. Soltis5 & Walter S. Judd5 1 Herbario EIF & Laboratorio de Sistemática y Evolución de Plantas, Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Universidad de Chile, Av. Santa Rosa 11315, La Pintana, Santiago, Chile 2 USDA-ARS-SHRS, National Germplasm Repository, 13601 Old Cutler Rd., Miami, Florida 33158, U.S.A. 3 Instituto de Botánica Darwinion, Labardén 200, CC 22, B1642HYD, San Isidro, Buenos Aires, Argentina 4 Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas, Postal Code 6109, 13083-970 Campinas, SP, Brazil 5 Florida Museum of Natural History, University of Florida, Gainesville, Florida 32611, U.S.A. Address for correspondence: Nicolás García, [email protected] DOI https://doi.org/10.1002/tax.12062 Abstract A robust generic classification for Amaryllidaceae has remained elusive mainly due to the lack of unequivocal diagnostic characters, a consequence of highly canalized variation and a deeply reticulated evolutionary history. A consensus classification is pro- posed here, based on recent molecular phylogenetic studies, morphological and cytogenetic variation, and accounting for secondary criteria of classification, such as nomenclatural stability. Using the latest sutribal classification of Hippeastreae (Hippeastrinae and Traubiinae) as a foundation, we propose the recognition of six genera, namely Eremolirion gen. nov., Hippeastrum, Phycella s.l., Rhodolirium s.str., Traubia, and Zephyranthes s.l. A subgeneric classification is suggested for Hippeastrum and Zephyranthes to denote putative subclades. -

Historical Survey of Easter Lily Name in Association with Lilium Longiflorum
® Floriculture and Ornamental Biotechnology ©2012 Global Science Books Historical Survey of Easter Lily Name in Association with Lilium longiflorum Eisuke Matsuo Professor Emeritus of Kyushu University, 1198-68, Tsuko, Ogori-shi 838-0102, Japan Correspondence : [email protected] ABSTRACT Based on the English dictionaries from around 1900, the author traces back how Lilium longiflorum came to be referred to as the Easter lily. The term “Easter lily” was given to any lily-like flowering species that bloomed around the Easter day, being called Easter flowers. Among them, L. candidum became the most famous one known as “Easter lily”. The evidence suggests that when more easily forcible bulb production began in the Bermuda Islands, the superiority of L. longiflorum over L. candidum in forcing ability and price, might have led to an explosive promotion of the usage of L. longiflorum as the Easter flower instead of L. candidum. Thus, the name "Easter lily" came to be transferred from L. candidum to L. longiflorum. _____________________________________________________________________________________________________________ Keywords: Easter decoration, Easter flower, Lilium candidum, Madonna lily CONTENTS INTRODUCTION.......................................................................................................................................................................................... 9 EASTER FLOWER AND EASTER LILY................................................................................................................................................... -

The Fossil Record of Angiosperm Families in Relation to Baraminology
The Proceedings of the International Conference on Creationism Volume 7 Article 31 2013 The Fossil Record of Angiosperm Families in Relation to Baraminology Roger W. Sanders Bryan College Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Sanders, Roger W. (2013) "The Fossil Record of Angiosperm Families in Relation to Baraminology," The Proceedings of the International Conference on Creationism: Vol. 7 , Article 31. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol7/iss1/31 Proceedings of the Seventh International Conference on Creationism. Pittsburgh, PA: Creation Science Fellowship THE FOSSIL RECORD OF ANGIOSPERM FAMILIES IN RELATION TO BARAMINOLOGY Roger W. Sanders, Ph.D., Bryan College #7802, 721 Bryan Drive, Dayton, TN 37321 USA KEYWORDS: Angiosperms, flowering plants, fossils, baramins, Flood, post-Flood continuity criterion, continuous fossil record ABSTRACT To help estimate the number and boundaries of created kinds (i.e., baramins) of flowering plants, the fossil record has been analyzed. To designate the status of baramin, a criterion is applied that tests whether some but not all of a group’s hierarchically immediate subgroups have a fossil record back to the Flood (accepted here as near the Cretaceous-Paleogene boundary). -
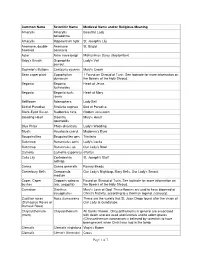
Of 7 Common Name Scientific Name Medieval Name And/Or Religious Meaning Amaryllis Amaryllis Belladonna Beautiful Lady
Common Name Scientific Name Medieval Name and/or Religious Meaning Amaryllis Amaryllis Beautiful Lady belladonna Amaryllis Hippeastrum hybr. St. Joseph's Lily Anemone, double- Anemone St. Brigid flowered coronaria Aster Aster nova-belgii Michaelmas Daisy (September) Baby's Breath Gypsophila Lady's Veil panicul. Bachelor's Buttons Centauria cyannis Mary's Crown Bean caper plant Zygophyllum ? Found on Shroud of Turin. See footnote for more information on dumosum the flowers of the Holy Shroud. Begonia Begonia Heart of Jesus fuchsioides Begonia Begonia fuch. Heart of Mary rosea Bellflower Adenophera Lady Bell Bird of Paradise Streliztia reginae Bird of Paradise Black-Eyed Susan Rudbeckia hirta Golden Jerusalem Bleeding Heart Dicentra Mary's Heart spectabilis Blue Phlox Phlox divaricata Lady's Wedding Bluets Houstonia caerul. Madonna's Eyes Bougainvillea Bougainvillea gen. Trinitaria Buttercup Ranunculus acris Lady's Locks Buttercup Ranunculus sp. Our Lady's Bowl Camelia Camellia (japonica) (Purity) Calla Lily Zantedeshia St. Joseph's Staff aethiop. Canna Canna generalis Rosary Beads Canterbury Bells Campanula Our Lady's Nightcap, Mary Bells, Our Lady's Smock medium Caper, Caper Capparis spinosa Found on Shroud of Turin. See footnote for more information on bushes (var. aegyptia) the flowers of the Holy Shroud. Carnation Dianthus Mary's Love of God. These flowers are said to have bloomed at caryophyllus Christ's Nativity, according to a German legend. (January) Castilian roses Rosa damascena These are the variety that St. Juan Diego found after the vision of (Damascus Roses or Our Lady at Guadalupe. Damask Rose) Chrysanthemum Chrysanthemum All Saints' Flower. Chrysanthemums in general are associated (mum) with death and are used and funerals and to adorn graves (Chrysanthemum coronarium is believed by scientists to have been present when Christ was laid in the tomb. -

LILIUM) PRODUCTION Faculty of Science, Department of Biology, University of Oulu
BIOTECHNOLOGICAL APPROACHES VELI-PEKKA PELKONEN IN LILY (LILIUM) PRODUCTION Faculty of Science, Department of Biology, University of Oulu OULU 2005 VELI-PEKKA PELKONEN BIOTECHNOLOGICAL APPROACHES IN LILY (LILIUM) PRODUCTION Academic Dissertation to be presented with the assent of the Faculty of Science, University of Oulu, for public discussion in Kuusamonsali (Auditorium YB210), Linnanmaa, on April 15th, 2005, at 12 noon OULUN YLIOPISTO, OULU 2005 Copyright © 2005 University of Oulu, 2005 Supervised by Professor Anja Hohtola Professor Hely Häggman Reviewed by Professor Anna Bach Professor Risto Tahvonen ISBN 951-42-7658-2 (nid.) ISBN 951-42-7659-0 (PDF) http://herkules.oulu.fi/isbn9514276590/ ISSN 0355-3191 http://herkules.oulu.fi/issn03553191/ OULU UNIVERSITY PRESS OULU 2005 Pelkonen, Veli-Pekka, Biotechnological approaches in lily (Lilium) production Faculty of Science, Department of Biology, University of Oulu, P.O.Box 3000, FIN-90014 University of Oulu, Finland 2005 Oulu, Finland Abstract Biotechnology has become a necessity, not only in research, but also in the culture and breeding of lilies. Various methods in tissue culture and molecular breeding have been applied to the production of commercially important lily species and cultivars. However, scientific research data of such species and varieties that have potential in the northern climate is scarce. In this work, different biotechnological methods were developed and used in the production and culture of a diversity of lily species belonging to different taxonomic groups. The aim was to test and develop further the existing methods in plant biotechnology for the developmental work and the production of novel hardy lily cultivars for northern climates. -

Easter Lily Growth and Development
Easter Lily Growth and Development 1985 Lot aClef - uNIPR TqTE .` cam V /rr sl 81. ,! l9' Technical Bulletin 148 AGRICULTURAL EXPERIMENT STATION Oregon State University Corvallis, Oregon Easter Lily Growth and Development ACKNOWLEDGMENTS We wish to acknowledge the help and encouragement of colleagues concernedwith lily bulb growing, handling, and forcing at Oregon State over the years. Faculty members including L. T.Blaney,O. C.Compton. Ralph Garren, L. H.Fuchigami, J. C.Green, P. J. Breen, and J. R. Stang have made important contributions to our knowledge of Lilium physiology. Graduate students in the Department of Horticulture have contributed much of thedetail to thisknowledge. These graduates include Vernon Clarkson (M.S. 1951),P. C. Lin(M.S. 1968). Dave Hartley (Ph.D.1968). S. Y. Wang(Ph.D.1969).Raj Bahadur(Ph.D. 1969), W. R. McCorkle(B.S. 1970, Missouri),K. D. Gray(M.S. 1974), B. J. Tomasovic(B.S.1974),and Y. T. Wang(M.S. 1981). We are especially grateful tothe FredC. Cloeckncr Foundation for its many years of financial support of the Easter lily research project and in preparation of this publication. The same is true for the Pacific Bulb Growers' Association, which has provided a field research station, a technician for its maintenance, graduate student support, and operating expenses at the station for more than 25 years. We would also mention the valuable contributions made by the Agricultural Extension Services of both Oregon and California through their able representatives Walt Schroeder (Curry County, Oregon) and John Lenz (Del Norte County, California). We are indebted to H. -

Ornamental Plants in Different Approaches
Ornamental Plants in Different Approaches Assoc. Prof. Dr. Arzu ÇIĞ cultivation sustainibility ecology propagation ORNAMENTAL PLANTS IN DIFFERENT APPROACHES EDITOR Assoc. Prof. Dr. Arzu ÇIĞ AUTHORS Atilla DURSUN Feran AŞUR Husrev MENNAN Görkem ÖRÜK Kazım MAVİ İbrahim ÇELİK Murat Ertuğrul YAZGAN Muhemet Zeki KARİPÇİN Mustafa Ercan ÖZZAMBAK Funda ANKAYA Ramazan MAMMADOV Emrah ZEYBEKOĞLU Şevket ALP Halit KARAGÖZ Arzu ÇIĞ Jovana OSTOJIĆ Bihter Çolak ESETLILI Meltem Yağmur WALLACE Elif BOZDOGAN SERT Murat TURAN Elif AKPINAR KÜLEKÇİ Samim KAYIKÇI Firat PALA Zehra Tugba GUZEL Mirjana LJUBOJEVIĆ Fulya UZUNOĞLU Nazire MİKAİL Selin TEMİZEL Slavica VUKOVIĆ Meral DOĞAN Ali SALMAN İbrahim Halil HATİPOĞLU Dragana ŠUNJKA İsmail Hakkı ÜRÜN Fazilet PARLAKOVA KARAGÖZ Atakan PİRLİ Nihan BAŞ ZEYBEKOĞLU M. Anıl ÖRÜK Copyright © 2020 by iksad publishing house All rights reserved. No part of this publication may be reproduced, distributed or transmitted in any form or by any means, including photocopying, recording or other electronic or mechanical methods, without the prior written permission of the publisher, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law. Institution of Economic Development and Social Researches Publications® (The Licence Number of Publicator: 2014/31220) TURKEY TR: +90 342 606 06 75 USA: +1 631 685 0 853 E mail: [email protected] www.iksadyayinevi.com It is responsibility of the author to abide by the publishing ethics rules. Iksad Publications – 2020© ISBN: 978-625-7687-07-2 Cover Design: İbrahim KAYA December / 2020 Ankara / Turkey Size = 16 x 24 cm CONTENTS PREFACE Assoc. Prof. Dr. Arzu ÇIĞ……………………………………………1 CHAPTER 1 DOUBLE FLOWER TRAIT IN ORNAMENTAL PLANTS: FROM HISTORICAL PERSPECTIVE TO MOLECULAR MECHANISMS Prof. -

20. LILIUM Linnaeus, Sp. Pl. 1: 302. 1753. 百合属 Bai He Shu Liang Songyun (梁松筠 Liang Song-Jun); Minoru N
Flora of China 24: 135–149. 2000. 20. LILIUM Linnaeus, Sp. Pl. 1: 302. 1753. 百合属 bai he shu Liang Songyun (梁松筠 Liang Song-jun); Minoru N. Tamura Herbs perennial, bulbiferous. Bulb of many imbricate, fleshy scales, without tunic. Stem erect, leafy. Leaves alternate, rarely whorled, sessile or subsessile, usually linear to linear-lanceolate. Bulblets sometimes present in leaf axils. Inflorescence terminal, a raceme or solitary flower, very rarely an umbel or corymb; bracts leaflike. Flowers often funnelform or campanulate, sometimes tub- ular or cupular. Tepals 6, free, usually connivent, sometimes strongly recurved or revolute, white, yellow, greenish, or reddish to pur- plish, nectariferous near base adaxially; nectaries usually narrowly grooved, sometimes fringed with papillae or hairs, rarely flat on outer tepals. Stamens 6; filaments subulate or filiform, sometimes pubescent; anthers dorsifixed, versatile. Ovary 3-loculed; ovules many per locule. Style elongate, slender; stigma swollen, usually 3-lobed. Fruit a loculicidal capsule. Seeds many, arranged like a pile of coins in each valve, flat, narrowly winged all round. About 115 species: temperate and alpine regions of the N hemisphere, especially in E Asia; 55 species (35 endemic, one introduced) in China. The status of Lilium puerense Y. Y. Qian (Guihaia 11: 125. 1991) and L. rockii R. H. Miao (Acta Scient. Nat. Univ. Sunyatseni 34(3): 81. 1995) is unclear. Lilium puerense was described from S Yunnan (Pu’er Xian), based on specimens collected in 1987 (holotype: Y. Y. Qian 1774, SMAO). It is said to be similar to L. sulphureum, but with leaf margin papillose, bracts ovate, and ovary greenish (vs. -

System Garden Masterplan, Melbourne University 2018
SYstem GARDEN LANDSCAPE MASTERPLAN STAGE 4 - MASTERPLAN FINAL REPORT 8th MARCH 2018 landscape architecture and GLAS urban design CONTENTS EXECUTIVE SUMMARY 1 INTRODUCTION 3 HistorY OF THE SYstem GARDEN 4 THE GARDEN TODAY 5 KEY ISSUES FACING THE SYstem GARDEN 6 Masterplan VISION 7 K EY VALUES 8 THE SYstem GARDEN AND OC21 9 VISION: A BOTANIC GARDEN FOR THE CAMPUS 10 MASTERPLAN PRINCIPLES 11 THE SYstem GARDEN MASTERPLAN 13 strategic INITIATIVES 15 BotanicAL DivERSiTy - SuB-cLASS PLANTiNG GuiDELiNES 16 INTERPRETATION StrateGY 17 UNIVERSITY HISTORY 18 INDIGENOUS ConnecTION 19 SUSTAINABILITY 20 MATERIALS PALETTE 21 MATERiALS PALETTE - LiGHTiNG AND PoWER 22 MATERiALS PALETTE - coNSoLiDATiNG SERvicES 23 FURNITURE 24 Access 25 ART AND EVENTS IN THE GARDEN 26 Masterplan ELEMENTS 27 master PLAN ELEMENTS 28 PERIMETER PATH AND EDGE SPACES 29 SYstem GARDEN GATEs 30 ENTRy AvENuES - BiZARRE SENTRiES 31 THE FORMAL GARDEN 32 WETLAND cANAL 37 THE INFORMAL GARDEN 38 COURTYARD GARDENS 43 rainforest GARDEN 44 FERN AND LICHEN COURTYARD 45 APOTHECARY GARDEN 47 RESEARCH GARDENS 48 implementation STAGING 50 APPENDIX 1: costing 55 APPENDIX 2: CONSULTANT REPORTS 57 EXecUTIVE SUMMARY IntroDUction The System Garden is a special space. Originally laid out in 1856 by Professor Frederick McCoy and The Core values, are key to the current and future operation of the Parkville campus, they have a • indigenous connection: the System Garden provides indigenous interpretation through Edward LaTrobe Bateman, it is a botanic garden configured specifically for learning. It provides a direct link to the University’s OC21 strategy (Our Campus in the 21st Century) and will drive the the Billibellary’s walk and stop within the System Garden. -

60 Uiz R. Prameela
UIZ Q R. PRAMEELA UN F 1. It gives sweetly scented 5. It is called ‘Blood lily’, fl owers and it also called blooms from May to July ‘Amazon lily’, blooms and it has been used as from November to a component of arrow January. poison and fi shing poison. a. Eucharis grandifl ora a. Hippeastrum b. Hymenocallis li oralis b. Amaryllis belladonna c. Zephyranthes grandifl ora c. Scadoxus mul fl orus d. Pancra um trifl orum d. Zephyranthes rosea 6. It means beau ful membrane, blooms in rainy season with white 2. This lily is na ve of fragrant fl owers. It is also South Africa, having long called ‘Spider lily’. leaves. It is also called ‘belladonna lily’ or ‘naked a. Proiphys alba lady’. It contains alkaloid b. Hymenocallis li oralis bellarmine. c. Eucharis grandifl ora a. Scadoxus mul fl orus d. Pancra um trifl orum b. Atropa belladonna c. Amaryllis belladonna d. Crinum asia cum 7. Zephyranthes species are collec vely called rain lilies or Fairy lilies, Zephyr-Flower or Thunder-Flower. They are ny fl owering plants ideal for edgings to borders. They are available in White, Pink, and Yellow. Which of the following is ‘White Zephyr’, blossoms in sunny days? 3. It is a popular indoor ornamental plant; commonly known as ‘Knight’s-star-lily’. a. Hippeastrum b. Lilium c. Crinum a. Zephyranthes candida b. Z. rosea d. Amaryllis c. Z. aurea d. Z. carinata 8. Iden fy the ‘pink Zephyr- 4. Leaves broadly ovate Flower’, it blossoms from to round and leathery. July to December.