Easter Lily Growth and Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Survey of Easter Lily Name in Association with Lilium Longiflorum
® Floriculture and Ornamental Biotechnology ©2012 Global Science Books Historical Survey of Easter Lily Name in Association with Lilium longiflorum Eisuke Matsuo Professor Emeritus of Kyushu University, 1198-68, Tsuko, Ogori-shi 838-0102, Japan Correspondence : [email protected] ABSTRACT Based on the English dictionaries from around 1900, the author traces back how Lilium longiflorum came to be referred to as the Easter lily. The term “Easter lily” was given to any lily-like flowering species that bloomed around the Easter day, being called Easter flowers. Among them, L. candidum became the most famous one known as “Easter lily”. The evidence suggests that when more easily forcible bulb production began in the Bermuda Islands, the superiority of L. longiflorum over L. candidum in forcing ability and price, might have led to an explosive promotion of the usage of L. longiflorum as the Easter flower instead of L. candidum. Thus, the name "Easter lily" came to be transferred from L. candidum to L. longiflorum. _____________________________________________________________________________________________________________ Keywords: Easter decoration, Easter flower, Lilium candidum, Madonna lily CONTENTS INTRODUCTION.......................................................................................................................................................................................... 9 EASTER FLOWER AND EASTER LILY................................................................................................................................................... -
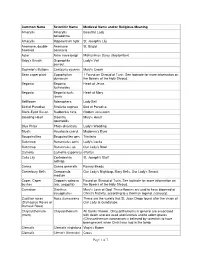
Of 7 Common Name Scientific Name Medieval Name And/Or Religious Meaning Amaryllis Amaryllis Belladonna Beautiful Lady
Common Name Scientific Name Medieval Name and/or Religious Meaning Amaryllis Amaryllis Beautiful Lady belladonna Amaryllis Hippeastrum hybr. St. Joseph's Lily Anemone, double- Anemone St. Brigid flowered coronaria Aster Aster nova-belgii Michaelmas Daisy (September) Baby's Breath Gypsophila Lady's Veil panicul. Bachelor's Buttons Centauria cyannis Mary's Crown Bean caper plant Zygophyllum ? Found on Shroud of Turin. See footnote for more information on dumosum the flowers of the Holy Shroud. Begonia Begonia Heart of Jesus fuchsioides Begonia Begonia fuch. Heart of Mary rosea Bellflower Adenophera Lady Bell Bird of Paradise Streliztia reginae Bird of Paradise Black-Eyed Susan Rudbeckia hirta Golden Jerusalem Bleeding Heart Dicentra Mary's Heart spectabilis Blue Phlox Phlox divaricata Lady's Wedding Bluets Houstonia caerul. Madonna's Eyes Bougainvillea Bougainvillea gen. Trinitaria Buttercup Ranunculus acris Lady's Locks Buttercup Ranunculus sp. Our Lady's Bowl Camelia Camellia (japonica) (Purity) Calla Lily Zantedeshia St. Joseph's Staff aethiop. Canna Canna generalis Rosary Beads Canterbury Bells Campanula Our Lady's Nightcap, Mary Bells, Our Lady's Smock medium Caper, Caper Capparis spinosa Found on Shroud of Turin. See footnote for more information on bushes (var. aegyptia) the flowers of the Holy Shroud. Carnation Dianthus Mary's Love of God. These flowers are said to have bloomed at caryophyllus Christ's Nativity, according to a German legend. (January) Castilian roses Rosa damascena These are the variety that St. Juan Diego found after the vision of (Damascus Roses or Our Lady at Guadalupe. Damask Rose) Chrysanthemum Chrysanthemum All Saints' Flower. Chrysanthemums in general are associated (mum) with death and are used and funerals and to adorn graves (Chrysanthemum coronarium is believed by scientists to have been present when Christ was laid in the tomb. -

20. LILIUM Linnaeus, Sp. Pl. 1: 302. 1753. 百合属 Bai He Shu Liang Songyun (梁松筠 Liang Song-Jun); Minoru N
Flora of China 24: 135–149. 2000. 20. LILIUM Linnaeus, Sp. Pl. 1: 302. 1753. 百合属 bai he shu Liang Songyun (梁松筠 Liang Song-jun); Minoru N. Tamura Herbs perennial, bulbiferous. Bulb of many imbricate, fleshy scales, without tunic. Stem erect, leafy. Leaves alternate, rarely whorled, sessile or subsessile, usually linear to linear-lanceolate. Bulblets sometimes present in leaf axils. Inflorescence terminal, a raceme or solitary flower, very rarely an umbel or corymb; bracts leaflike. Flowers often funnelform or campanulate, sometimes tub- ular or cupular. Tepals 6, free, usually connivent, sometimes strongly recurved or revolute, white, yellow, greenish, or reddish to pur- plish, nectariferous near base adaxially; nectaries usually narrowly grooved, sometimes fringed with papillae or hairs, rarely flat on outer tepals. Stamens 6; filaments subulate or filiform, sometimes pubescent; anthers dorsifixed, versatile. Ovary 3-loculed; ovules many per locule. Style elongate, slender; stigma swollen, usually 3-lobed. Fruit a loculicidal capsule. Seeds many, arranged like a pile of coins in each valve, flat, narrowly winged all round. About 115 species: temperate and alpine regions of the N hemisphere, especially in E Asia; 55 species (35 endemic, one introduced) in China. The status of Lilium puerense Y. Y. Qian (Guihaia 11: 125. 1991) and L. rockii R. H. Miao (Acta Scient. Nat. Univ. Sunyatseni 34(3): 81. 1995) is unclear. Lilium puerense was described from S Yunnan (Pu’er Xian), based on specimens collected in 1987 (holotype: Y. Y. Qian 1774, SMAO). It is said to be similar to L. sulphureum, but with leaf margin papillose, bracts ovate, and ovary greenish (vs. -

60 Uiz R. Prameela
UIZ Q R. PRAMEELA UN F 1. It gives sweetly scented 5. It is called ‘Blood lily’, fl owers and it also called blooms from May to July ‘Amazon lily’, blooms and it has been used as from November to a component of arrow January. poison and fi shing poison. a. Eucharis grandifl ora a. Hippeastrum b. Hymenocallis li oralis b. Amaryllis belladonna c. Zephyranthes grandifl ora c. Scadoxus mul fl orus d. Pancra um trifl orum d. Zephyranthes rosea 6. It means beau ful membrane, blooms in rainy season with white 2. This lily is na ve of fragrant fl owers. It is also South Africa, having long called ‘Spider lily’. leaves. It is also called ‘belladonna lily’ or ‘naked a. Proiphys alba lady’. It contains alkaloid b. Hymenocallis li oralis bellarmine. c. Eucharis grandifl ora a. Scadoxus mul fl orus d. Pancra um trifl orum b. Atropa belladonna c. Amaryllis belladonna d. Crinum asia cum 7. Zephyranthes species are collec vely called rain lilies or Fairy lilies, Zephyr-Flower or Thunder-Flower. They are ny fl owering plants ideal for edgings to borders. They are available in White, Pink, and Yellow. Which of the following is ‘White Zephyr’, blossoms in sunny days? 3. It is a popular indoor ornamental plant; commonly known as ‘Knight’s-star-lily’. a. Hippeastrum b. Lilium c. Crinum a. Zephyranthes candida b. Z. rosea d. Amaryllis c. Z. aurea d. Z. carinata 8. Iden fy the ‘pink Zephyr- 4. Leaves broadly ovate Flower’, it blossoms from to round and leathery. July to December. -

The Root Systems of Onion and Allium Fistulosum in The
Flower development of Lilium longiflorum: Characterisation of MADS-box transcription factors VAGNER AUGUSTO BENEDITO Promotor: Prof. dr. S. C. de Vries Hoogleraar in de Biochemie Wageningen Universiteit Co-promotoren: Dr. F. A. Krens Clusterleider, businessunit Biodiversiteit en Veredeling, Plant Research International Prof. dr. Ir. G. C. Angenent Clusterleider, businessunit Bioscience, Plant Research International / Hoogleraar in de Moleculaire Plantontwikkeling, Katholieke Universiteit Nijmegen Promotiecommissie: Dr. L. Colombo Università delgi Studi di Milano, Italië Prof. dr. T. Bisseling Wageningen Universiteit Dr. R. G. H. Immink Plant Research International Prof. dr. L. H. W. van der Plas Wageningen Universiteit Flower development of Lilium longiflorum: Characterisation of MADS-box transcription factors Vagner Augusto Benedito Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit Prof. dr. ir. L. Speelman in het openbaar te verdedigen op woensdag 14 januari 2004 des namiddags te vier uur in de Aula Flower development of Lilium longiflorum: Characterisation of MADS-box transcription factors. Ph.D. thesis, Wageningen University, Wageningen, The Netherlands 2004. With summaries in English, Dutch and Portuguese. ISBN: 90-5808-947-9 "To the people from Brazil, I dedicate this thesis" Por isso na impaciência Desta sede de saber, Como as aves do deserto As almas buscam beber… Oh! Bendito o que semeia Livros… livros à mão cheia… E manda o povo pensar! O livro caindo n'alma É germe -

Interspecific Lily Hybrids with the Ability to Flower Precociously and to Produce Multiple Flower Stalks from Lilium Formosanum
J. Japan. Soc. Hort. Sci. 77 (3): 312–317. 2008. Available online at www.jstage.jst.go.jp/browse/jjshs1 JSHS © 2008 Interspecific Lily Hybrids with the Ability to Flower Precociously and to Produce Multiple Flower Stalks from Lilium formosanum Hiroki Saruwatari1, Yuka Shuto-Nakano1, Kanehiro Nakano1, Michikazu Hiramatsu2*, Yukio Ozaki2 and Hiroshi Okubo2 1Graduate School of Bioresource and Bioenvironmental Sciences, Kyushu University, Fukuoka 812-8581, Japan 2Faculty of Agriculture, Kyushu University, Fukuoka 812-8581, Japan Lilium formosanum Wallace has remarkable traits such as ‘precocious flowering’ ability, i.e., it reaches anthesis within 12 months from seed germination, with multiple shooting of flower stalks. To verify the possibility of the usefulness of these traits in lily breeding, nine combinations of interspecific crosses (L. formosanum as the seed parent; L. auratum, L. speciosum, L. regale, ‘Lollypop’, ‘Pink Tiger’, ‘Zaza’, ‘Le Reve’, ‘Marco Polo’, and ‘African Queen’ as pollen parents) were carried out with cut-style pollination and ovary-slice culture. Germination was observed in all nine interspecific crosses and 53 hybrids were obtained. Thirty (56.6%) of the 53 hybrids and two self-pollinated progenies of L. formosanum reached anthesis within 24 months from germination through ovary- slice culture. Multiple shooting of flower stalks was recognized in 11 (36.7%) of those flowered hybrids. Four hybrids, the pollen parents of which were Asiatic hybrid lilies with colored flowers, expressed ‘precocious flowering’ ability, multiple shooting of flower stalks, and entirely colored flowers simultaneously. These results suggest the possibility of breeding new types of cultivars with triple favorable traits from the cross between L. -

Forty Days and Forty Nights
APRIL 2021 FORTY DAYS AND FORTY NIGHTS FORDINGBRIDGE AND RINGWOOD PARISH MAGAZINE In This Edition: The Great Bridge at Fordingbridge • Fr Paul Says: • Book Reviews (Penny Sharp and Gabriel Gamble) • Poetry Please G W Carryl, Robert Browning, Geoffrey Chaucer) • Easter Gardening (Sheila Wade) • The Invisible Times (Margaret Fraser) • The Great Bridge at Fordingbridge (Text Ed, picture George Shepperdley) • Salisbury Cathedral Tower Tour (continued, Chris Basham) • The Anni Rosler Letters (Provided by David Saunders) • Growing Up in Fordingbridge (George Shepperdley • Rohingya in Bangladesh (Helen Eales) • Cookery Corner (Janet Arden) • End Bits (Ed) Fr. Paul Says… This is a photograph of rather lovely oil painting by Henry Woodward which my old Canadian friend George Shepperdley (see below) has on his bedroom wall. George thinks it was painted in the 1970s, but I think it was earlier than that as there is no footbridge. I remember the footbridge being added because I rowed beneath that bridge practically every day until 1962! Of course, the artist may have just left it out! April begins this year with our celebration of the ‘Easter Triduum’, on the 1st, 2nd and 3rd April. The The view is looking downstream from the meadow just upstream of what is now Caxtons, but was then The Riverside Triduum consists of three days making up the Bakery. On the left is Dr. Vickery’s garden, then the bridge itself with The George, as ever, on the bank to the South single liturgy of the Paschal Mystery or the life, with the Albany Hotel and its river frontage adjoining the Greyhound Hotel garden and boathouse. -
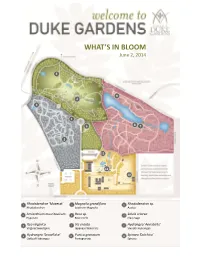
What's in Bloom
WHAT’S IN BLOOM June 2, 2014 5 9 4 3 1 6 8 7 2 12 11 10 1 Rhododendron ‘Maxecat’ 5 Magnolia grandiflora 9 Rhododendron sp. Rhododendron Southern Magnolia Azalea 2 Amianthium muscitoxicum 6 Rosa sp. 10 Salvia sclarea Flypoison Rose Circle Clary Sage 3 Itea virginica 7 Iris ensata 11 Hydrangea ‘Annabelle’ Virginia Sweetspire Japanese Water Iris Smooth Hydrangea 4 Hydrangea ‘Snowflake’ 8 Punica granatum 12 Spiraea ‘Dolchica’ Oakleaf Hydrangea Pomegranate Spiraea WHAT’S IN BLOOM June 2, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Galax urceolata Beetleweed Agarista populifolia Florida Hobblebush Hydrangea quercifolia Oakleaf Hydrangea Aquilegia canadensis Columbine Penstemon digitalis Foxglove Beardtongue Amianthium muscitoxicum Flypoison Rhododendron ‘Maxecat’ Chrysogonum virginianum Green and Gold Saururus cernuus Lizard’s Tail Coreopsis verticillata Whorled Tickseed Thalictrum dasycarpum Purple Meadow-rue Cotinus obovatus American Smoketree Euonymus americana Bursting-heart DORIS DUKE CENTER GARDENS Rehmannia elata Chinese Foxglove Crinum ‘Carolina Beauty’ Crinum Rhododendron sp. Azalea Gaura lindheimeri Gaura Spiraea x bumalda ‘Dolchica’ Spiraea Hydrangea arborescens ‘Annabelle’ Smooth Valeriana officinalis Valerian Hydrangea Verbena bonariensis Tall Verbena Hydrangea quercifolia Oakleaf Hydrangea Viburnum awabuki ‘Chindo’ Chindo Viburnum Itea virginica Virginia Sweetspire Nepeta ‘Walker’s Low’ Catmint CULBERSON ASIATIC ARBORETUM Rhododendron sp. Azalea Cladrastis sinensis Chinese Yellow-wood Rehmannia elata Chinese Foxglove Cornus kousa Kousa -

Flowers of Liliaceae and Related Families Grown in Southern Arizona Gardens
Flowers of Liliaceae and related families grown in southern Arizona gardens Item Type text; Thesis-Reproduction (electronic) Authors Emery, Eleanor Merrill, 1911- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 25/09/2021 04:44:06 Link to Item http://hdl.handle.net/10150/551139 Flowers of Liliaceae and Related Families Grown in Southern Arizona Gardens by Eleanor Merrill Emery Submitted in partial fulfillment of the requirements for the degree of Master of Science in the Graduate College University of Arizona 19 3 6 Approved: iajor pro / 936, U, 7 Abstract A careful botanical study has been made of numerous rare or little-known bulbous flowers of chiefly the Lil- iales Order, growing on the campus of the University of Arizona and in southern Arizona gardens. Correct scientific names as well as the common names are included. Seasons of planting and growth, methods of culture, and soils best suited to their successful growth have been recorded. 105572 The writer wishes to express her gratitude to Professor John J. Thornher for his assistance in the preparation of this work. Introduction This is a study of the Lily and related families, growing in gardens of southern Arizona and the Southwest generally. The flowers are easily recognized by their narrow, parallel-veined leaves and flower parts which are in threes or sixes. -

Flowers of Our Lady for Southwest Arizona Cultivation
Flowers of Our Lady for Southwest Arizona Cultivation Botanical Name Type Hardiness Heat Common Name Religious Name Association SS Rec A B P H/T Z H 13 XER Sub-Tropical Perennials, Shrubs Acanthus mollis P SH 7--11 12--7 S X Bear's-Breech Angel's Wing Heaven Artemisia vulgaris P T 13--15 12--1 S Artemisia St. John's Girdle FeastofSt.JohnBapt. Asparagus densiflorus P T 13--15 12--1 S X Asparagus Fern Mary's Tresses Crucifixion Bougainvillea buttiana CL T 13--15 12--1 S X Madonna Madonna Nativity R Bougainvillea glabra CL T 13--15 12--1 S X Bougainvillea Trinitaria Annunciation Canna indica P SH 8--11 12--1 S Canna Lily Rosary Bead Plant Rosary Dahlia hybrida P T 9--11 12--1 S Garden Dahlia Church Flower Pentecost Dalea spinosa T T 9--11 12--1 S Smoke Tree Christ's Crown Crowning with thorns Erodium cicutarium P T 14--15 12--10 X Pin Clover Madonna's Pins Nazareth R Euphorbia pulcherrima. P T 13--15 12-10 S Poinsettea Nativity Flower Nativity R Euporbia splendens p T 11--12 12--1 S Crown-of-Thorns Christ's Crown Crowningwiththorns R Fuchsia hybrida P T 9--11 12--9 X Fuchsia Lady's Eardrops Annunciation Gaillardia acauis P SH 8--10 12--1 ? Angelita Daisy Angelita Angels Hesperocallis undulata p T 9--10 10--9 ? Desert Lily Mary's Flower (Purity) Annunciation R Hibiscus rosa-sinensis P T 14--15 12--1 S Tropical Hybiscus Christ's Bood Crucifixion Kalanchoe blossfeldiana P T 11--15 12--1 X Kalancho ? Lantana camara P T 9--10 12--1 S X Lantana Christ's Plant Nazareth R Nerium oleander P T 13--15 12--1 S X Oleander Rose of Jericho Nativity Nicotiana -

Taxonomy and Phylogeny of the Genus Lilium
® Floriculture and Ornamental Biotechnology ©2012 Global Science Books Taxonomy and Phylogeny of the Genus Lilium Veli-Pekka Pelkonen* • Anna-Maria Pirttilä Department of Biology/Botany, University of Oulu, P.O. Box 3000, FIN-90014 Oulu, Finland Corresponding author : * [email protected] ABSTRACT Lilies have a long history as ornamental plants. Today, there is an ever increasing variety of new lily cultivars due to the significant progress in the propagation and development of new methods in breeding. The domesticated native species have retained their place along with new hybrids in commercialized horticultural industry, and they have sustained their invaluable potential for the breeding of new cultivars for garden use as well as for greenhouse culture. Systematics has always played an important role in plant breeding, giving guidelines for hybridization, although biotechnology has introduced new solutions for many problems that were evolutionary obstacles especially in inter-specific crossings before. The genus Lilium has been a subject of variable suggestions for classification systems, and the process still continues. The currently accepted concept for the phylogenetic and taxonomic system for all species is based on geographical, structural and genetic information. In our review, we give an insight into the latest progress in revealing the taxonomical relationships within the genus. According to the existing GenBank sequence data, we have constructed a phylogenetic tree consisting of the main species and sections of the genus. Provided with species photos, the tree gives a brief overview of phylogeny- and morphology- based classifications, which are not always congruent. In the tree mainly all species grouped into sections defined within the genus, but L. -

INTRODUCTION.--Black Scale of Easter Lily (Lilium Longiflorum Thunb.) Bulbs Was Found in the United States for the First Time in 1937
Plant Pathology Circular No. 33 Florida Dept. of Agriculture March 1965 Division of Plant Industry Revised July 1980 BLACK SCALE OF LILY BULBS E. K. Sobers INTRODUCTION.--Black scale of Easter lily (Lilium longiflorum Thunb.) bulbs was found in the United States for the first time in 1937. Commercial bulb growers in several southern Louisiana parishes experienced heavy losses because the bulbs, blackened by disease, were unsalable. Although the disease was reported from Bermuda in 1895, its cause remained unknown until 1944, when Plakidas (1) described Colletotrichum lilii Flak, as the causal organism. HOST RANGE.--Results of pathogenicity tests conducted in 1962 (3), revealed that bulbs of Creole, Georgia (Fig. 1), Croft, and Estate Easter lilies were susceptible to C. lilii. The fungus was also found to be pathogenic to bulbs of L. amabile Palibin, L. dauricum Ker var. sanguineum, L. martagon L. ('Album' ), L. pumilum DC., and L. umbe11atum Hort. Fig. 1. Healthy (left) and diseased (right) Georgia and Creole Easter lily bulbs. DISTRIBUTION.--Black scale was known to occur only in Bermuda and Louisiana until recent pathogenicity tests proved its presence in Florida. These occurrences, however, have been traced directly to bulbs imported from Japan. Suspected cases of black scale from Alabama, California, China, India, Japan, and Oregon were investigated between 1950 and 1955. In each instance a Colletotrichum indistinguishable from C. lilii was isolated. Extensive pathogenicity tests proved all isolates incapable of inciting black scale lesions on Easter lily bulbs or on bulbs of the species from which they were originally isolated (2). DISEASE SYMPTOMS.—Initially lesions are light brown, irregular, slightly depressed, and found mostly on the apical half of the outer scale surface.