Field Research Laboratories for Trace Metal Ecotoxicology Cambridge.Org/Mbi Philip S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Notes on the Parish of Mylor, Cornwall
C.i i ^v /- NOTES ON THE PARISH OF MYLOR /v\. (crt MVI.OK CII r RCII. -SO UIH I'OKCil AND CROSS O !• ST. MlLoKIS. [NOTES ON THE PARISH OF MYLOR CORNWALL. BY HUGH P. OLIVEY M.R.C.S. Uaunton BARNICOTT &- PEARCE, ATHEN^UM PRESS 1907 BARNICOTT AND PEARCE PRINTERS Preface. T is usual to write something as a preface, and this generally appears to be to make some excuse for having written at all. In a pre- face to Tom Toole and his Friends — a very interesting book published a few years ago, by Mrs. Henry Sandford, in which the poets Coleridge and Wordsworth, together with the Wedgwoods and many other eminent men of that day figure,—the author says, on one occasion, when surrounded by old letters, note books, etc., an old and faithful servant remon- " " strated with her thus : And what for ? she " demanded very emphatically. There's many a hundred dozen books already as nobody ever reads." Her hook certainly justified her efforts, and needed no excuse. But what shall I say of this } What for do 1 launch this little book, which only refers to the parish ot Mylor ^ vi Preface. The great majority of us are convinced that the county of our birth is the best part of Eng- land, and if we are folk country-born, that our parish is the most favoured spot in it. With something of this idea prompting me, I have en- deavoured to look up all available information and documents, and elaborate such by personal recollections and by reference to authorities. -

Environmental Protection 1990 Classification Of
NRA National Rivers Authority ENVIRONMENTAL PROTECTION 1990 CLASSIFICATION OF ESTUARY QUALITY TWS/92/005 Author: J. Driver Tidal Waters Scientist GORDON H BIELBY BSc Regional General Manager C V M Davies Environmental Protection Manager 1990 CLASSIFICATION OF ESTUARY QUALITY Introduction 1. Each year, 22 estuaries in the region are classified in terms of their aesthetic, chemical and biological quality. In 1990 two surveys were carried out. Hie first (or "DoE Survey") was a repeat of the subjective assessment made in 1989 and previous years. This was based on the current NWC system of points allocation approved by the DoE/NWC Standing Technical Advisory Committee on Water Quality (Appendix 1). The second (or "NRA Survey") used the chemical data collected in each estuary during 1990 to provide a more objective classification for each estuary (using the same points allocation system as above). 2. Every 5 years, the NWC classification is reported to the DoE to form part of the report of the national survey of river and estuary quality. This was last done in 1985. The 1990 NWC classification has been forwarded to the DoE. 3. The results of the NRA survey will be forwarded to NRA HQ and will form the basis of future work. 1990 Classification i) DoE Survey 4. The subjective assessment of each estuary in the region follows that of previous years. Appendix 2 give details of the 1990 classification. 5. For each estuary 2 lengths (1985 length and 1990 length) are reported. Towards the end of 1989 all the estuaries were re-measured (by splitting each estuary into sections, or zones, and measuring each zone). -

MYLOR MAGAZINE November 2012
Your free magazine — please take one MYLOR MAGAZINE November 2012 Serving the whole community Mylor Magazine [email protected] Published by: Mylor Community Publications Group Trustees: Chris Perkins (Chairman) Revd Roger Nicholls (Secretary) David Eastburn Editor: Michael Jeans-Jakobsson 01326 374767 Deputy Editor: Malcolm Clark Community & Advertising: Val Jeans-Jakobsson 01326 374767 Photography: Geoff Adams 01326 374197 Treasurer: Andy Goodman 01326 373975 Editorial Team: Judy Menage (PCC) Roger Deeming Printing: Leaflet Express 01872 865744 Publication date is the 1st of the month Deadline date for Copy is 15th of previous month Advertising in Mylor Magazine Rates — per issue (artwork supplied) Full page (A5) £16.00 Half page £10.00 Quarter page £6.00 E-mail: [email protected] for further details Cover: A home-built boat nears completion in Mylor. See article on page 26. (photo Michael JJ) 2 Contents 4 Vicar’s letter 17 Mylor Singers 5 Church notes 19 Health and Fitness 6 Church news 20 Village Snippets 7 Flower Club 20 Tremayne Hall supporters 8 Xmas gift fair 21 Local History - Ukrainians 8 Garden Club 24 Centre spread 11 Fish and Chips 26 Local exploits - building Iola 11 Local History Group 31 Farming notes 13 Photo competition 33 Wildwatch 14 Truro DFAS 37 On the water 14 Book Group 43 Crossword 15 Mylor Sessions 44 More About - Jan Sadler 16 Mylor Movies 47 Crossword solution 17 Duchy Opera 47 Monthly cartoon Village Diary November December 3 6.30pm Scouts & Guides bonfire 1 TH 10-12 Xmas Fair FSM 5 OS 7.30pm MFC demonstration 3 7pm Xmas Lights switch-on 7 Castaway’s first quiz night see p45 8 TH 7pm con Mylor Singers p17 10 TH 7.30 con Treverva MV Choir 9 MC 4pm Christingle service 10 PH 10-12 CM Vera welcomes you 10 OS 7.30 MGC Lake District 12 OS 7.30pm MGC talk see p8 12 TL 7pm Christmas Pie 12 TH 7pm MS John Williams see p15 12 TH MM 7:30 Hope Springs 12 OS 7.30 MGC Trees for small gdns. -

Ref: LCAA6651 Guide £425,000
Ref: LCAA6651 Guide £425,000 Creekview, Goonvrea, Perranarworthal, Nr. Truro, Cornwall FREEHOLD An exciting opportunity to acquire a brand new, highly individual architect designed reverse level contemporary home, occupying a superb elevated location at the end of a private no-through lane enjoying fantastic far reaching views down the valley to the head of the Restronguet Creek. Built to an exacting standard and to a high degree of specification with fantastic 3/4 bedroomed accommodation plus double garage, parking, low maintenance gardens and use of 7 acres of communal woodland. 2 Ref: LCAA6651 SUMMARY OF ACCOMMODATION Ground Floor: entrance hall, master bedroom with en-suite bathroom, 2 further double bedrooms, family shower room, airing cupboard. First Floor: study/bedroom 4, kitchen opening to dining/sitting room, south facing balcony. Lower Ground Floor: double garage/utility. Outside: low maintenance south facing gardens. Additional garden area plus additional parking. Underground bike store. DESCRIPTION • A fantastic brand new highly individual contemporary reverse level home. • Cedar clad and smooth rendered elevations with gas centrally heated double glazed accommodation designed and supervised by Nigel Bush of NHB Architects. Benefitting from a new build architects certificate warranty. • Occupying a wonderful elevated location set towards the end of an exclusive private no-through road and enjoying fantastic far reaching views down the wooded valley below to the head of Restronguet Creek. A vista which must be seen first hand to be fully appreciated. • Built to a high degree of specification and designed to make best use of the views. The living space includes a high quality German Mobila kitchen with integrated Neff appliances. -
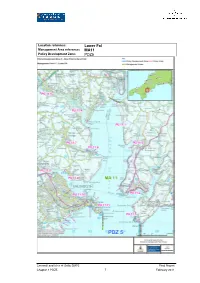
MA11 Policy Development Zone: PDZ5
Location reference: Lower Fal Management Area reference: MA11 Policy Development Zone: PDZ5 Cornwall and Isles of Scilly SMP2 Final Report Chapter 4 PDZ5 7 February 2011 DISCUSSION AND DETAILED POLICY DEVELOPMENT The preferred plan for the Lower Fal aims to balance the provision of support to the core settlements of Falmouth, Penryn, St Mawes, St Just, Flushing, Restronguet and Mylor (in line with the high-level SMP objectives) with a management approach which does not adversely impact on the undeveloped parts of the lower estuary and importantly takes account of any potential impacts on the Fal & Helford SAC. It is important to note that there is a legal requirement to not adversely affect the integrity of the SAC; through impacts such as the loss of intertidal feeding areas by not allowing the high water mark to move inland due to climate change. This, of course, requires a number of different policy options to be employed at different locations. From a high level view-point, it can be seen that across the whole Management Area (and indeed across the entire estuary system) there will be a trend toward a reduction in intertidal area due to sea level rise. Wherever the landward movement of MHWS is constrained by the rising topography or defences, reduction in intertidal area may occur. It is likely that a net overall reduction in intertidal area may occur toward the latter part of the SMP timeframe when considering sea level rise in isolation. However the picture is actually much more complex than this – erosion, accretion, sedimentation, changes in the tidal prism, increases in rainfall and fluvial flow will also affect the current pattern of intertidal exposure. -

CREEK WATERS Restronguet Point, Feock, Nr
CREEK WATERS Restronguet Point, Feock, Nr. Truro, South Cornwall • Exclusive waterside peninsula. CREEK WATERS • West facing with magnificent creek views. RESTRONGUET POINT, FEOCK, NR. TRURO, • 31’ part double height reception hall with hardwood and glass staircase. SOUTH CORNWALL • A superb 23’9” x 23’6” living room with wonderful panoramic water views and Feock • Truro 5 miles • Falmouth 9 miles • St Mawes 3 miles by water fitted study off. Distances approximate. • Wonderful 31’ family room and kitchen with extensive immaculate units, granite A stunning detached marine residence of very high quality worksurfaces, appliances and fabulous water views. and impeccable presentation offering 4 en-suite bedroomed • 20’ principal bedroom with vaulted ceiling, balcony and Villeroy & Boch en-suite accommodation of contemporary design with superb ground floor bathroom. 3 further double bedrooms – each with Villeroy & Boch en-suite living and entertaining space. facilities. 2 Electric gated driveway, garaging and extensive /3 of an acre • Large double garage. gardens. Exceptional stunning west facing panoramic views • Secure electric gated driveway and plenty of car/boat parking space. spanning Restronguet Creek, the wooded creek bank and rolling 2 countryside. • Beautiful extensive /3 acre landscaped gardens and grounds facing the water. In every sense, an outstanding family home in one of the most prestigious locations in Cornwall between Restronguet Creek and the Fal Estuary – some of the finest day sailing waters in the UK. SUMMARY OF ACCOMMODATION Ground Floor: 31’ reception hall – part double height with hardwood and glass staircase, cloakroom/wc, fine living room and study 23’9” x 23’6” max overall, stunning family room and kitchen almost 30’ x 15’. -

County Wildlife Sites Criteria for Cornwall Appendices
Heading County Wildife Site Criteria for Cornwall Appendices Environmental Records Centre for Cornwall and the Isles of Scilly Appendix 1 List of County Wildlife Sites in Cornwall List current at July 2010 PENWITH P/K 1 Hayle Estuary and River System P1.1 Hayle Estuary P1.3 Treloweth Woods P1.4 St Erth Pools P/K 1.5 Relubbus Ponds P1.6 Carbismill to Relubbus P/K 2 North Coast P2.2 Great Moor Zawn to Porthmeor Cove P2.5 Towednack Quae Head to Clodgy Point P/K 2.7 Hayle Dune System P3 South Coast P3.1 Prussia Cove to Stackhouse Cove P3.2 Stackhouse Cove to Perran Sands P3.3 Marazion Marsh P3.4 Mount's Bay P3.5 Mousehole to Lamorna Cove P3.6 Lamorna Cove to Merthen Point P3.7 Merthen Point to Porthcurno P3.8 Porthcurno to Porthgwarra P3.9 Porthgwarra to Pendower Coves P3.10 Pendower Coves to Pordenack Point P3.11 Pordenack Point to Sennen Cove P3.12 Sennen Cove to Carn Gloose P/K 4 Red River Valley P/K 4.1 Lower Red River P5 Gwinear Tips and Trungle Valley P6.2 Clodgy Moor P7 Cold Harbour Marsh P8 Drift Reservoir P9 Higher and Lower Hill Woods(includes Trencrom Hill) P10 Selena Moor P10.1 West Selena Moor P10.2 East Selena Moor P11 Penwith Moors P11.1 Carn Brea, Tredinney & Bartinney Commons P11.2 Caer Bran and Sancreed Beacon P11.3 Carnyorth Common and Bostraze Bog P11.4 Chun Downs to Boswens Common P11.5 Boswarva Carn P11.6 Central Moors P11.7 Churchtown Common to Trendrine Hill P11.8 Rosewall Hill P11.9 Bussow Moor & Carn Stabba P11.10 Busvargus & Tregeseal Common to Dowran Common & Bosworlas Moor P11.11 Botrea Downs P11.12 Bosvenning -

Ref: LCAA1820
Ref: LCAA6241 £409,950 Higher Tregoose Barn, Point Road, Devoran, Truro, Cornwall FREEHOLD In a glorious semi rural setting in rolling countryside just above the sailing waters of Restronguet Creek; a superb deceptively spacious semi-detached 3/4 bedroomed mellow stone barn conversion of immense quality. With well appointed accommodation on three floors, sheltered south facing gardens, garage and parking in a sought after and highly convenient location. 2 Ref: LCAA6241 SUMMARY OF ACCOMMODATION Ground Floor: entrance hall with turning staircase to first floor, master bedroom and 2 further double bedrooms, family bathroom, shower room. First Floor: landing with airing cupboard, wc, kitchen/dining room, sitting room with contemporary woodburning stove, turning staircase to 2nd floor. Second Floor: attic room/occasional bedroom 4. Outside: sheltered level lawned gardens, parking for 4 cars, garage with rear utility area plus sail loft. Wood stores. DESCRIPTION Beautiful mellow stone barn conversion with cut granite quoins and lintels with natural slate roof. An imposing and highly attractive building converted circa 28 years ago. Glorious semi rural location set back from a quiet lane in beautiful rolling countryside above the sailing waters of Restronguet Creek. Deceptively spacious accommodation on three floors with high ceilings, well presented having been much improved by the current owners. Reverse level accommodation with fantastic recently refitted kitchen/dining room with space for 8 seater dining table, large sitting room with contemporary woodburning stove and oblique views to Restronguet Creek. 3 Ref: LCAA6241 3 ground floor double bedrooms plus second floor attic room/study/occasional bedroom 4. Sheltered south facing gardens bounded by fields, gated driveway with parking for 4 cars plus detached garage with utility and sail loft. -

Fal and Helford Csac
Characterisation of European Marine Sites The Fal and Helford (candidate) Special Area of Conservation Marine Biological Association Occasional publication No. 8 Cover photograph: Mike Cudlipp, Twinbrook Falmouth Site Characterisation of the South West European Marine Sites Fal and Helford cSAC W.J. Langston∗1, B.S.Chesman1, G.R.Burt1, S.J. Hawkins1, J.Readman2 and 3 P.Worsfold April 2003 A study carried out on behalf of the Environment Agency and English Nature by the Plymouth Marine Science Partnership ∗ 1 (and address for correspondence): Marine Biological Association, Citadel Hill, Plymouth PL1 2PB (email: [email protected]): 2Plymouth Marine Laboratory, Prospect Place, Plymouth; 3PERC, Plymouth University, Drakes Circus, Plymouth ACKNOWLEDGEMENTS Thanks are due to members of the steering group for advice and help during this project, notably, Mark Taylor and Roger Covey of English Nature and Nicky Cunningham, Peter Jonas and Roger Saxon of the Environment Agency (South West Region). The helpful contributions of other EN and EA personnel are also gratefully acknowledged. It should be noted, however, that the opinions expressed in this report are largely those of the authors and do not necessarily reflect the views of EA or EN. © 2003 by Marine Biological Association of the U.K., Plymouth Devon All rights reserved. No part of this publication may be reproduced in any form or by any means without permission in writing from the Marine Biological Association. ii Plate 1: Some of the operations/activities which may cause disturbance or deterioration -

Sgw05 Mylor Creek to Restronguet Creek
walkitcornwall SELF GUIDED WALKS Walk no:- Distance Degree of difficulty - Terrain SGW05 Miles/kms Easy to moderate Views of Mylor Creek and then over to Messack Point Walk name 5 miles Circular or linear and St Just once you reach Mylor Creek (8 kms) the River Fal. All on a flat Restronguet creek Circular riverside path with an ascent and descent inland to finish. Grid ref start point OS Explorer Map Number 803 361 Circular 105 Grid ref finish point Brief description highlighting character and degree of difficulty of the walk One of the most peaceful walks starting from Mylor Creek to Restronguet Creek starting from Mylor Bridge around the Greatwood quay onto the famous Pandora Inn. Estuary birds should be in abundance and a path that hugs the riverside. Public transport information Nearest Toilets and Nearest Disabled Toilets Public toilets at Mylor Bridge Nearest Car parks and Nearest Car Parks with disabled provision Car parks in Mylor bridge Nearest refreshments Mylor Bridge and Pandora Inn at Restronguet Passage Further information 1. Fal River Information on Prince of Wales Quay, Falmouth https://www.facebook.com/walkitcornwall https://twitter.com/walkitcornwall Detailed description highlighting character of the walk, what to look for etc Starting at Mylor Bridge keep to the north side of Mylor Creek by walking down the side road next to the large grocery store. A small quay/parking area is a place to stop and check for birdlife just after the small post office. Follow the concrete path and there is a footpath to your left going uphill. -

Building Plot with Direct Waterfrontage and Stunning
Ref: LCAA5766 Offers in excess of £850,000 Building plot at Creek Bank, Restronguet Point, Feock, Truro, Cornwall FREEHOLD Feock ● Truro 5 miles ● Falmouth 9 miles ● St Mawes 3 miles by water – distances approximate. BUILDING PLOT WITH DIRECT WATERFRONTAGE AND STUNNING FAR REACHING WESTERLY VIEWS On Restronguet Point – one of the best kept secrets on the south coast of Britain – a beautiful slender peninsula beside the Fal Estuary, accessed by a no-through lane and home to some of the most desirable waterfronting residences in Cornwall. On the much sought after ‘sunny’ side of ‘The Point’; an exceptionally rare opportunity to acquire A GREEN FIELD BUILDING PLOT WITH FULL VALID PLANNING CONSENT to construct a stunning brand new waterfronting house which will command idyllic views over Restronguet Creek and beautiful countryside beyond. In all, approximately 0.4 of an acre extending down to a landing stage with ownership to mean high water and about 60ft direct waterfrontage. An important opportunity to secure one of the very best waterfronting building plots for sale in any location in Cornwall, let alone on the immensely sought after western side of ‘The Point’ which is home to some of the most valuable properties in the county. 2 Ref: LCAA5766 SUMMARY OF PROPOSED ACCOMMODATION Planning consent has been obtained for the construction of a new dwelling with further approval under application PA14/07847 for a non- material amendment to PA13/01358 for repositioning of the double garage adjacent to the new dwelling and omission of the carport. We understand that the proposed accommodation is to extend to almost 300sq. -

Morvoren Trolver Croft, Feock, South Cornwall
MORVOREN TROLVER CROFT, FEOCK, SOUTH CORNWALL MORVOREN TROLVER CROFT, FEOCK, TRURO, SOUTH CORNWALL Located on one of the most prestigious roads in Cornwall and with excellent direct waterfrontage, including ownership of the foreshore and moorings; a large detached 5 bedroomed house facing west over its own ½ an acre of garden and across Restronguet Creek to countryside beyond. A spectacularly located marine home in one of the very best plots along this road, enjoying an excellent level of privacy, slipway and outstanding views to Point Quay, in a convenient position close to Truro and the sailing waters of the Carrick Roads. SUMMARY OF ACCOMMODATION Ground Floor: reception hall, wc, study, living room, conservatory, dining room, kitchen, utility room, bedroom 4, second wc, guest sitting room/playroom, guest bedroom 5. First Floor: landing, 3 bedrooms (1 en-suite), bath/shower room. Outside: large westerly facing lawned gardens, slipway, moorings, direct waterfrontage and ownership of foreshore. Driveway, double garage, various stores and workshops, boat shed, 1 bedroomed timber chalet. In all, about ½ an acre. Viewing strictly by appointment through the vendors’ Sole Agents: Lillicrap Chilcott . Landrian House . 59-60 Lemon Street Truro . TR1 2PE Tel: 01872 273473 Fax: 01872 273474 Email: [email protected] www.waterfrontandcountryhomes.com DESCRIPTION The house is offered in an excellent decorative state, having more immediately Trolver Croft are for many, the perfect Morvoren is found in one of the largest and best positioned been well cared for and maintained. A large entrance hall with location. plots along Trolver Croft, one of south Cornwall’s most highly staircase rising to the first floor, leads to most of the ground floor rooms with a study and fourth bedroom at the front of Towards the mouth of Restronguet Creek are deep water regarded private roads.