LEUSTATIN (Cladribine) Injection Should Be Administered Under the Supervision of a Qualified Physician Experienced in the Use of Antineoplastic Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Application of a Characterized Pre-Clinical
THE APPLICATION OF A CHARACTERIZED PRE-CLINICAL GLIOBLASTOMA ONCOSPHERE MODEL TO IN VITRO AND IN VIVO THERAPEUTIC TESTING by Kelli M. Wilson A dissertation submitted to Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland March, 2014 ABSTRACT Glioblastoma multiforme (GBM) is a lethal brain cancer with a median survival time (MST) of approximately 15 months following treatment. A serious challenge facing the development of new drugs for the treatment of GBM is that preclinical models fail to replicate the human GBM phenotype. Here we report the Johns Hopkins Oncosphere Panel (JHOP), a panel of GBM oncosphere cell lines. These cell lines were validated by their ability to form tumors intracranially with histological features of human GBM and GBM variant tumors. We then completed whole exome sequencing on JHOP and found that they contain genetic alterations in GBM driver genes such as PTEN, TP53 and CDKN2A. Two JHOP cell lines were utilized in a high throughput drug screen of 466 compounds that were selected to represent late stage clinical development and a wide range of mechanisms. Drugs that were inhibitory in both cell lines were EGFR inhibitors, NF-kB inhibitors and apoptosis activators. We also examined drugs that were inhibitory in a single cell line. Effective drugs in the PTEN null and NF1 wild type cell line showed a limited number of drug targets with EGFR inhibitors being the largest group of cytotoxic compounds. However, in the PTEN mutant, NF1 null cell line, VEGFR/PDGFR inhibitors and dual PIK3/mTOR inhibitors were the most common effective compounds. -

Synthesis of Ribavirin, Tecadenoson, and Cladribine by Enzymatic Transglycosylation
catalysts Article Synthesis of Ribavirin, Tecadenoson, and Cladribine by Enzymatic Transglycosylation 1, 2 2,3 2 Marco Rabuffetti y, Teodora Bavaro , Riccardo Semproli , Giulia Cattaneo , Michela Massone 1, Carlo F. Morelli 1 , Giovanna Speranza 1,* and Daniela Ubiali 2,* 1 Department of Chemistry, University of Milan, via Golgi 19, I-20133 Milano, Italy; marco.rabuff[email protected] (M.R.); [email protected] (M.M.); [email protected] (C.F.M.) 2 Department of Drug Sciences, University of Pavia, viale Taramelli 12, I-27100 Pavia, Italy; [email protected] (T.B.); [email protected] (R.S.); [email protected] (G.C.) 3 Consorzio Italbiotec, via Fantoli 15/16, c/o Polo Multimedica, I-20138 Milano, Italy * Correspondence: [email protected] (G.S.); [email protected] (D.U.); Tel.: +39-02-50314097 (G.S.); +39-0382-987889 (D.U.) Present address: Department of Food, Environmental and Nutritional Sciences, University of Milan, y via Mangiagalli 25, I-20133 Milano, Italy. Received: 7 March 2019; Accepted: 8 April 2019; Published: 12 April 2019 Abstract: Despite the impressive progress in nucleoside chemistry to date, the synthesis of nucleoside analogues is still a challenge. Chemoenzymatic synthesis has been proven to overcome most of the constraints of conventional nucleoside chemistry. A purine nucleoside phosphorylase from Aeromonas hydrophila (AhPNP) has been used herein to catalyze the synthesis of Ribavirin, Tecadenoson, and Cladribine, by a “one-pot, one-enzyme” transglycosylation, which is the transfer of the carbohydrate moiety from a nucleoside donor to a heterocyclic base. As the sugar donor, 7-methylguanosine iodide and its 20-deoxy counterpart were synthesized and incubated either with the “purine-like” base or the modified purine of the three selected APIs. -

COMPARISON of the WHO ATC CLASSIFICATION & Ephmra/Intellus Worldwide ANATOMICAL CLASSIFICATION
COMPARISON OF THE WHO ATC CLASSIFICATION & EphMRA/Intellus Worldwide ANATOMICAL CLASSIFICATION: VERSION June 2019 2 Comparison of the WHO ATC Classification and EphMRA / Intellus Worldwide Anatomical Classification The following booklet is designed to improve the understanding of the two classification systems. The development of the two systems had previously taken place separately. EphMRA and WHO are now working together to ensure that there is a convergence of the 2 systems rather than a divergence. In order to better understand the two classification systems, we should pay attention to the way in which substances/products are classified. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, e.g., NAPROXEN tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only. The purposes of classification are also different: The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA/Intellus Worldwide classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies. Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems. -
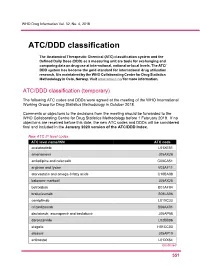
ATC/DDD Classification
WHO Drug Information Vol. 32, No. 4, 2018 ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international, national or local levels. The ATC/ DDD system has become the gold standard for international drug utilization research. It is maintained by the WHO Collaborating Centre for Drug Statistics Methodology in Oslo, Norway. Visit www.whocc.no/ for more information. ATC/DDD classification (temporary) The following ATC codes and DDDs were agreed at the meeting of the WHO International Working Group for Drug Statistics Methodology in October 2018. Comments or objections to the decisions from the meeting should be forwarded to the WHO Collaborating Centre for Drug Statistics Methodology before 1 February 2019. If no objections are received before this date, the new ATC codes and DDDs will be considered final and included in the January 2020 version of the ATC/DDD Index. New ATC 5th level codes ATC level name/INN ATC code acalabrutinib L01XE51 amenamevir J05AX26 amlodipine and celecoxib C08CA51 arginine and lysine V03AF11 atorvastatin and omega-3 fatty acids C10BA08 baloxavir marboxil J05AX25 betrixaban B01AF04 brolucizumab S01LA06 cemiplimab L01XC33 crizanlizumab B06AX01 daclatasvir, asunaprevir and beclabuvir J05AP58 darolutamide L02BB06 elagolix H01CC03 elbasvir J05AP10 entinostat L01XX64 Continued 5 51 ATC/DDD classification WHO Drug Information Vol. 32, No. 4, 2018 New ATC 5th level codes (continued) -

Cladribine Injection
PRODUCT MONOGRAPH PrCLADRIBINE INJECTION Solution for Intravenous Injection 1 mg/mL USP Antineoplastic / Chemotherapeutic Agent Fresenius Kabi Canada Ltd. Date of Revision: 165 Galaxy Blvd, Suite 100 June 9, 2015 Toronto, ON M9W 0C8 Control No.: 181078 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION ......................................................... 3 SUMMARY PRODUCT INFORMATION ............................................................................... 3 INDICATIONS AND CLINICAL USE ..................................................................................... 3 CONTRAINDICATIONS .......................................................................................................... 3 WARNINGS AND PRECAUTIONS ......................................................................................... 4 ADVERSE REACTIONS ........................................................................................................... 7 DRUG INTERACTIONS ......................................................................................................... 10 DOSAGE AND ADMINISTRATION ..................................................................................... 11 OVERDOSAGE ....................................................................................................................... 13 ACTION AND CLINICAL PHARMACOLOGY ................................................................... 14 STORAGE AND STABILITY ................................................................................................ -

Original Article Treatment of Hairy Cell Leukemia with Cladribine
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Annals of Oncology 13: 1641–1649, 2002 Original article DOI: 10.1093/annonc/mdf272 Treatment of hairy cell leukemia with cladribine (2-chlorodeoxyadenosine) by subcutaneous bolus injection: a phase II study A. von Rohr1*, S.-F. H. Schmitz2, A. Tichelli3, U. Hess4, D. Piguet5, M. Wernli6, N. Frickhofen7, G. Konwalinka8, G. Zulian9, M. Ghielmini10, B. Rufener2, C. Racine1, M. F. Fey1, T. Cerny1, D. Betticher1 & A. Tobler11 On Behalf of the Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland 1Institute of Medical Oncology, Inselspital, Bern; 2SIAK Coordinating Center, Bern; 3Division of Hematology, Kantonsspital, Basel; 4Department of Internal Medicine, Kantonsspital, St Gallen; 5Department of Internal Medicine, Hôpital des Cadolles, Neuchâtel; 6Department of Internal Medicine, Kantonsspital, Aarau, Switzerland; 7Department of Internal Medicine, University Hospital, Ulm, Germany; 8Department of Internal Medicine, University Hospital, Innsbruck, Austria; 9Department of Internal Medicine, University Hospital, Geneva; 10Oncology Institute of Southern Switzerland, Bellinzona; 11Central Hematology Laboratory, University Hospital, Bern, Switzerland Received 8 January 2002; revised 11 April 2002; accepted 24 April 2002 Background: To assess the activity and toxicity of 2-chlorodeoxyadenosine (cladribine, CDA) given by subcutaneous bolus injections to patients with hairy cell leukemia (HCL). Patients and methods: Sixty-two eligible patients with classic or prolymphocytic HCL (33 non- pretreated patients, 15 patients with relapse after previous treatment, and 14 patients with progressive disease during a treatment other than CDA) were treated with CDA 0.14 mg/kg/day by subcutaneous bolus injections for five consecutive days. -

Cladribine (Leustatin®) Pronounced: “KLAD-Rih-BEAN”
Cladribine Chemotherapy: Cladribine (Leustatin®) Pronounced: “KLAD-rih-BEAN” How drug is given: By vein (IV) Purpose: To treat hairy cell leukemia, non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and other cancers Things that may occur during or within hours of treatment • Facial flushing (warmth or redness of the face), itching, or a skin rash could occur. These symptoms are due to an allergic response and should be reported to your cancer care team right away. Things that may occur days to weeks after drug is given • Your blood cell counts may drop. This is known as bone marrow suppression. This includes a decrease in your: o Red blood cells, which carry oxygen in your body to help give you energy o White blood cells, which fight infection in your body o Platelets, which help clot the blood to stop bleeding This may happen 7 to 14 days after the drug is given and then blood counts should return to normal. If you have a fever of 100.5°F (38°C) or higher, chills, a cough, or any bleeding problems, tell your cancer care team right away. • Some patients may feel very tired, also known as fatigue. You may need to rest or take naps more often. Mild to moderate exercise can also be helpful in maintaining your energy. • Some patients may have mild nausea. You may be given medicine to help with this. • You may get a headache. Please talk to your doctor or nurse about what you can take for this. • In rare cases, nerves can be affected by this medicine. -
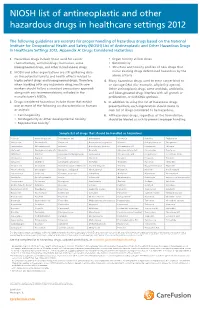
NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012
NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2012 The following guidelines are excerpts for proper handling of hazardous drugs based on the National Institute for Occupational Health and Safety (NIOSH) List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012, Appendix A: Drugs Considered Hazardous 1. Hazardous drugs include those used for cancer • Organ toxicity at low doses* chemotherapy, antiviral drugs, hormones, some • Genotoxicity** bioengineered drugs, and other miscellaneous drugs. • Structure and toxicity profiles of new drugs that 2. NIOSH and other organizations are still gathering data mimic existing drugs determined hazardous by the on the potential toxicity and health effects related to above criteria highly potent drugs and bioengineered drugs. Therefore, 4. Many hazardous drugs used to treat cancer bind to when working with any hazardous drug, health care or damage DNA (for example, alkylating agents). workers should follow a standard precautions approach Other antineoplastic drugs, some antivirals, antibiotics, along with any recommendations included in the and bioengineered drugs interfere with cell growth or manufacturer’s MSDSs. proliferation, or with DNA synthesis. 3. Drugs considered hazardous include those that exhibit 5. In addition to using the list of hazardous drugs one or more of the following six characteristics in humans presented here, each organization should create its or animals: own list of drugs considered to be hazardous. • Carcinogenicity 6. All hazardous -

Cladribine in the Treatment of Langerhans Cell Histiocytosis
Review Article iMedPub Journals Journal of Rare Disorders: Diagnosis & Therapy 2019 http://wwwimedpub.com ISSN 2380-7245 Vol. 5 No. 1:1 DOI: 10.36648/2380-7245.5.1.193 Cladribine in the Treatment of Langerhans Marlies E.H.M. van Hoef* Cell Histiocytosis: A Summary of the Literature Corresponding Author: Marlies E.H.M. van Hoef Abstract [email protected] Langerhans cell Histiocytosis (LCH) is a rare disease for which treatment with 2- chlorodeoxyadenosine (cladribine) has changed disease outcome. Cladribine has meanwhile been administered over two decades and is available for intravenous Citation: Van Hoef MEHM (2019) Cladribine and subcutaneous administration. Cladribine has been administered in treatment in the Treatment of Langerhans Cell of therapy naïve, relapsed and refractory LCH and induces high response rates, Histiocytosis: A Summary of the Literature. regardless of prior treatment. Literature describing the use of cladribine either J Rare Disorders Diagnosis & Therapy. Vol. 5 alone or in combination with cytarabine is summarized herein. No. 1: 1. Keywords: Cladribine; Langerhans cell histiocytosis; Summary Received: February 28, 2019; Accepted: March 14, 2019; Published: March 21, 2019 Introduction in central nervous system involvement, regardless whether there are tumorous lesions or neurodegenerative disease [4]. LCH is a rare disease involving clonal proliferation of Langerhans Cladribine (2-chlorodeoxyadenosine) is a purine analog that cells, which are abnormal cells derived from the bone marrow, was developed in the 1970s. It was first tested in humans in along with eosinophils, macrophages, lymphocytes and the early 1980s and became an established product for the multinucleated giant cells. The incidence is 2 to 10 cases per treatment of Langerhans cell histiocytosis (LCH). -

Treatment Selection That Works! the Ex Vivo Analysis of Programmed Cell Death EVA-PCD® Functional Profile
With Cancer, the Difference Between Life and Death May Simply Be an Informed Decision Treatment Selection That Works! The Ex Vivo Analysis of Programmed Cell Death EVA-PCD® FUNCTIONAL PROFILE CLINICAL APPLICATION Responders Distinguish Responders from Non-responders Non-Responders WHEN YOU THINK BIOPSY, THINK EVA-PCD 1 2 3 4 5 Fresh Micro- Drug Analysis & Report in tumor spheroids Exposure calibration seven days 1 cm or > u isolated u u u BENEFITS OF EVA-PCD t Apoptosis • Significantly improved response t Necrosis • Improved time to progression • Potential to diminish toxicity t Apoptosis tNecrosis Published results of cell death assays in Clinical application of assay-directed therapy common human solid tumor malignancies* (Performance characteristics calculated from the aggregate results) Disease n Overall Positive Negative Literature Response Response Fold Response Predictive Predictive reported rate for assay rate for assay advantage, Rate Accuracy Accuracy response (+) patients (-) patients assay (+) vs rate assay (-) Breast 194 64.9% 82.9% 88.9% 10% 46.0% 2.6% 17.6 Colon 54 16.6% 80.0% 97.7% 30% 77.0% 9.3% 8.3 NSCLC 47 29.7% 66.7% 93.1% 50% 88.9% 19.3% 4.6 Gyn 345 56.2% 77.0% 87.9% 70% 94.8% 35.8% 2.6 SCLC 19 26.0% 50.0% 84.6% TOTAL 659 50.6% 78.4% 90.1% * References available upon request. COMPARE RESULTS IN NSCLC Non Small Cell Lung Cancer Standard EVA-PCD Therapy p Value Response 64.5% 30.0% p = 0.00015 Survival Time to Progression 8.5 Months 6.0 Months Median Time in Months Survival 21.3 Months 12.5 Months Product-Limit Estimate -
Acute Myeloid Leukemia (AML) Treatment Regimens
Acute Myeloid Leukemia (AML) Treatment Regimens Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced healthcare team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are only provided to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data becomes available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. uInduction Therapy1 Note: All recommendations are Category 2A unless otherwise indicated. The NCCN believes the best option for any patient with cancer is in a clinical trial and strongly encourages this option for all patients. PATIENT CRITERIA REGIMEN AND DOSING Age <60 years2-8 Days 1–3: An anthracycline (daunorubicin 60–90mg/m2 IV OR idarubicin 12mg/m2) Days 1–7: Cytarabine 100–200mg/m2 continuous IV (Category 1). -

Chronic Myelogenous Leukemia (CML) Agents – Unified Formulary
bmchp.org | 888-566-0008 wellsense.org | 877-957-1300 Pharmacy Policy Chronic Myelogenous Leukemia (CML) Agents – Unified Formulary Policy Number: 9.709 Version Number: 2.0 Version Effective Date: 9/1/2021 Product Applicability All Plan+ Products Well Sense Health Plan Boston Medical Center HealthNet Plan New Hampshire Medicaid MassHealth- MCO MassHealth- ACO Qualified Health Plans/ConnectorCare/Employer Choice Direct Senior Care Options Note: Disclaimer and audit information is located at the end of this document. Prior Authorization Policy Reference Table: Drugs that require PA No PA Bosulif® (bosutinib) PD Gleevec® # (imatinib) Iclusig® (ponatinib) Sprycel® (dasatinib) Synribo® (omacetaxine mepesuccinate) Tasigna® (nilotinib) # This is a brand-name drug with FDA “A”-rated generic equivalents. Prior authorization is required for the brand, unless a particular form of that drug (for example, tablet, capsule, or liquid) does not have an FDA “A”-rated generic equivalent. PD Preferred Drug. In general, MassHealth requires a trial of the preferred drug or clinical rationale for prescribing a non-preferred drug within a therapeutic class. Please note, for CML agents, a trial with a preferred agent is not required prior to approval of a non-preferred agent. + Plan refers to Boston Medical Center Health Plan, Inc. and its affiliates and subsidiaries offering health coverage plans to enrolled members. The Plan operates in Massachusetts under the trade name Boston Medical Center HealthNet Plan and in other states under the trade name Well Sense Health Plan. Chronic Myelogenous Leukemia (CML) Agents 1 of 6 Procedure: Approval Chronic Myelogenous Leukemia (Bosulif®, Iclusig® Synribo® (omacetaxine Diagnosis: mepesuccinate)) Acute Lymphoblastic Leukemia (Iclusig®) Approval Criteria: Prescriber provides documentation of ALL of the following: 1.