Experience with the Urinary Tetrasaccharide Metabolite for Pompe Disease in the Diagnostic Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of Total Dietary Fibre and Available Carbohydrates: a Rapid Integrated Procedure That Simulates in Vivo Digestion
860 DOI 10.1002/star.201500017 Starch/Stärke 2015, 67, 860–883 RESEARCH ARTICLE Determination of total dietary fibre and available carbohydrates: A rapid integrated procedure that simulates in vivo digestion Barry V. McCleary, Naomi Sloane and Anna Draga Megazyme International Ireland, Bray Business Park, Bray, County Wicklow, Ireland The new definition of dietary fibre introduced by Codex Alimentarius in 2008 includes resistant Received: January 23, 2015 starch and the option to include non-digestible oligosaccharides. Implementation of this definition Revised: March 5, 2015 required new methodology. An integrated total dietary fibre method was evaluated and accepted by Accepted: March 5, 2015 AOAC InternationalandAACCInternational(AOACMethods2009.01and2011.25;AACCMethod 32–45.01 and 32–50.01, and recently adopted by Codex Alimentarius as a Type I Method. However, in application of the method to a diverse range of food samples and particularly food ingredients, some limitations have been identified. One of the ongoing criticisms of this method was that the time of incubation with pancreatic a-amylase/amyloglucosidase mixture was 16 h, whereas the time for food to transit through the human small intestine was likely to be approximately 4 h. In the current work, we use an incubation time of 4 h, and have evaluated incubation conditions that yield resistant starch and dietary values in line with ileostomy results within this time frame. Problems associated with production, hydrolysis and chromatography of various oligosaccharides have been addressed resulting in a more rapid procedure that is directly applicable to all foods and food ingredients currently available. Keywords: Available carbohydrates / Codex Alimentarius / Dietary fibre determination / Enzymic / Non-digestible oligosaccharides / Resistant starch : Additional supporting information may be found in the online version of this article at the publisher’s web-site. -
Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(Glcnach Tetrasaccharide
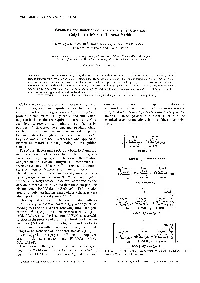
Polymer Journal, Vol. 30. No. 8, pp 653-658 ( 1998) Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(GlcNAch Tetrasaccharide Kazukiyo KoBAYASHI,t Shoko KAMIYA, Minoru MATSUYAMA,* Takeomi MURATA,* and Taichi Usur* Graduate School of' Engineering, Nagoya University, Chikusa, Nagoya 464-8603, Japan * Faculty of' Agriculture, Shizuoka University, Ohya, Shizuoka 422---8529, Japan (Received February 16, 1998) ABSTRACT: Tetrasaccharide Galfil--+4(GlcNAch was synthesized from N,N',N"-triacetylchitotriose (GlcNAc)s and lactose using transglycosylation with a P-o-galactosidase from Bacillus circu/ans. The reducing terminal of Galfil--+4(GlcNAclJ was oxidized and connected to p-vinylbenzylamine via amide linkage, and the resulting oligosaccharide-substituted styrene monomer was polymerized with the radical initiator, 2,2' -azobis(2-amidinopropane) dihydrochloride at 60"C. Glycopolystyrene was found to bind strongly with wheat germ agglutinin (WGA) and tomato (Lycopersicon esculentum) agglutinin (LEA) by inhibition of hemagglutination and double diffusion. KEY WORDS Oligosaccharides / Glycopolymers / Lectins / Enzymatic Synthesis/ Recognition / Cell surface carbohydrates from glycoproteins, glyco dimensional immunodiffusion in agar and inhibition of lipids, proteoglycans, and capsular polysaccharides play lectin-induced hemagglutination. Comparison was made important roles in biological events, 1.2 Carbohydrate using analogous homopolymers carrying N-acetyllactos protein interaction is usually weak and multivalent amine and chitooligosaccharides. Chart I illustrates the oligosaccharide chains are required to target cell surface chemical structures and abbreviations of these glycopoly carbohydrate receptors and inhibit host infection by mers. pathogens. 3 Glycopolymers carrying pendant oligo saccharide chains can be regarded as multivalent ligands known to interact strongly with lectins and antibodies, 4 · 5 Glycopolymers have been synthesized and applied as biomedical materials having biological recognition signals. -

Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments
A Dissertation Titled: Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments from B. fragilis ATCC 25285/NCTC 9343 with Alternating Charges on Adjacent Monosaccharides by Pradheep Eradi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry ___________________________________________ Dr. Peter R. Andreana, PhD, Committee Chair ___________________________________________ Dr. Steve Sucheck, PhD, Committee Member ___________________________________________ Dr. Jianglong Zhu, PhD, Committee Member ___________________________________________ Dr. Amanda C. Bryant-Freidrich, PhD, Committee Member ___________________________________________ Dr. Cyndee Gruden, Dean College of Graduate Studies The University of Toledo May 2019 Copyright 2019 Pradheep Eradi This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments from B. fragilis ATCC 25285/NCTC 9343 with Alternating Charges on Adjacent Monosaccharides by Pradheep Eradi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry The University of Toledo May 2019 Zwitterionic polysaccharides (ZPSs) are a relatively new class of carbohydrate antigens, with a paradigm shifting property; they can activate CD4+ T-cells in the absence of lipids, peptide(s) or protein(s) upon MHC class II presentation. Up until now, various anaerobic bacteria are known to express ZPSs, for example, PS A1, PS A2 and PS B (Bacteroides fragilis), Sp1 (Streptococcus pneumoniae), CP5 and CP8 (Staphylococcus aureus) and O-chain antigen (Morganella morgani). Among all the afore mentioned ZPSs, Sp1 and PS A1 polysaccharides were the prime focus of research for the past few decades and their biological properties are very well-understood. -

Tetrasaccharide Glycoforms from Bacillus Anthracis Exosporium and Fragments Thereof
molecules Article Structure-Immunogenicity Relationship of α- and β-Tetrasaccharide Glycoforms from Bacillus anthracis Exosporium and Fragments Thereof Riccardo De Ricco 1, Christy L. Ventura 2, Filippo Carboni 1, Rina Saksena 3,†, Pavol Kováˇc 3 and Roberto Adamo 1,* ID 1 GSK, Research Centre, via Fiorentina 1, 53100 Siena, Italy; [email protected] (R.D.R.); fi[email protected] (F.C.) 2 Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; [email protected] 3 NIDDK, LBC, National Institutes of Health, Bethesda, MD 20892-0815, USA; [email protected] (R.S.); [email protected] (P.K.) * Correspondence: [email protected]; Tel.: +39-0577-539393 † Current address: Department of Chemistry, The University of New Mexico, Albuquerque, NM 87131, USA. Received: 12 July 2018; Accepted: 17 August 2018; Published: 20 August 2018 Abstract: The tetrasaccharide (2-O-methyl-4-(3-hydroxy-3-methylbutamido)-4,6-dideoxy-α-D- glucopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!2)-L-rhamnopyranose) from the major exosporium protein (BclA) of Bacillus anthracis has been proposed as a target for development of diagnostics and immune therapy or prophylaxis. While the immunodominant character of the anthrose residue has been previously elucidated, the role of the stereochemical configuration of the downstream rhamnose is unknown. Because the linkage of this residue to the GlcNAc bridging the glycan and the protein is lost during isolation of the tetrasaccharide, its α- and β-glycoforms have been synthesized. Herein, we prepared neoglycoconjugates from a series of fragments of the tetrasaccharide, including the complete α- and β-tetrasaccharide glycoforms, a 2-demethoxylated version of the α-tetrasaccharide, and the α- and β-trirhamnosides and CRM197. -

Quantitative Changes in Various Sugar Concentrations During Fermentation
QUAriTri'AT:CVE GHAI'jGES 111 VARIOUS SUGAR GOIjCm'RATlOKS UJRIMG FEH'IiSm'ATlOII Oi<' DOUGliS, SPONGiiS AMD BRliT/ZS ^' Ting Jui Tan^ 3. S., Tai-.v-an Provincial ChTJungshing Universitj . A MSTEK'S THESIS subnitted in partial fulfilLTient of the req-uireiaent Tor the decree MSTitlR OF SCIENCE Food Science Depairtinsnt Ox Grain Science arid Ladiistiy KAIIS'IS STATK UNXVilRSI'i'Y Manhattan, Kansas 1963 ApTrov'i'id by: I'lajor Professor . ' - LD . /?(? . tSX table of coiitehts eitroduction 1 REVIEV7 OF LITER/iTUPJi; 2 I. The Carbohydrates of Ivlieat and VJheat Flour 2 II. Quantitative Determination of Sugars in Natural ............ Materials 5 III, The Fate of Sugars in Breadmaking ......... 11 ^lETHOD AND MATERIALS l6 I. Flour Samples l6 II. Formulation, Dough I-Iake-up and Balcing 16 III, Determination of Gas Production Rates 19 IV. Schedule of Sample Collection for Sugar Analyses ..... 20 V. Method of Ejctraction of Sugars from Dough, Brew, Sponge and Bread Crumb . , 21 VI. tiethods of Analyses , 21 RESULTS AND DISCUSSION 25 I. of Discussion the Experimental Methods , 25 II. Change in Total Reducing Sugars of the Extracts 31 III. Change of Concentration of Individual Sugars During Fermentation ........... ....... 32 IV. Rate of Gas Production 34 V. Residual Sugars in Bread 36 SU>2^SIRY AND CONCLUSION 37 ACKI^Oi-niEDGEMEIT Zfo LITERATURE CITED , ^ i^l ii LIST _0F TABLES I. The I-Iono- and Di- saccharide Content of Flour , 3 II, Changes of Sugar Content in Steeped and Germinated Ivheat , , , , 12 III, Size of Sar.ples Used in Detenrdnation of Gas Production Hates , . 19 IV. -

Nutritive Sweeteners from Corn Have Become America’S Premier Sweeteners
NutritiveNutritive SweetenersSweeteners FromFrom CornCorn CONTENTS Member Companies and Plant Locations ....................................... 2 Foreword .......................................................................................... 3 Historical Perspective ...................................................................... 4 Research and development orientation ....................................... 5 Technology aimed at needs .......................................................... 7 Growth, Development and Diversity ............................................. 7 CONTENTS Classification and Nutrition ............................................................ 9 Classification ................................................................................. 9 Corn sweeteners in nutrition ..................................................... 10 Technical Background ................................................................... 11 Corn starch ................................................................................. 11 Starch hydrolysis ........................................................................ 13 Crystalline dextrose .................................................................... 14 Dextrose isomerization .............................................................. 15 Manufacture ................................................................................... 17 Corn syrups ................................................................................ 17 Dried corn syrups ...................................................................... -

Isolation and Characterization of a Blood Group A-Specific Urinary Tetrasaccharide
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Volume 119, number 1 FEBS LETTERS September 1980 ISOLATION AND CHARACTERIZATION OF A BLOOD GROUP A-SPECIFIC URINARY TETRASACCHARIDE Christian DERAPPE+. Arne LUNDBLAD*, Lisbeth MESSETER and Sigfrid SVENSSON Department of Clinical Chemistry, University Hospital, S-221 85 Lund, Sweden Received 21 July 1980 1. Introduction also collected from a blood group A secretor without any dietary restrictions. All urines were stored at Blood group A-, B- and H-active tetra- en penta- -20°C until required. The 6 h urine portions were saccharides have been isolated from urine of starved deionized by passage through a mixed-bed ion- ABH secretors [ 1,2]. Ingestion of free galactose or a exchange resin. To 20 ml of the desalted urine 200 pg glycoside of galactose (lactose) induces the formation isomaltotetraose were added as internal standard and and excretion of blood group specific di- and tri- the sample was reduced and permethylated. The saccharides [3,4] and small amounts of hexa- and permethylated oligosaccharides were analyzed by heptasaccharides [4]. The serological activities of GLC-MS [6]. some A- and B-specific oligosaccharides have been Isolation of the A-active tetrasaccharide was car- studied and compared with those of oligosaccharides ried out using gel chromatography on Sephadex G-l 5 from soluble blood group A and B substances [3,5]. (for experimental details see legend to fig.l), and When urine from individuals of different ABO blood preparative paper chromatography was done on group and secretor status was analyzed by gas-liquid Whatman no. -

Monosaccharidic Mimetics of the Sialyl Lewis Tetrasaccharide Based on 2
Issue in Honor of Prof. Rainer Beckert ARKIVOC 2012 (iii) 134-148 Monosaccharidic mimetics of the sialyl LewisX tetrasaccharide based on 2,7-dihydroxynaphthalene Stefan Weck,a,b Martin Frank,c Alf Hamann,d and Till Opatz*a,b a Institute of Organic Chemistry, University of Mainz, Duesbergweg 10-14, 55128 Mainz, Germany. b Department of Chemistry, University of Hamburg, Martin-Luther-King-Platz 6, 20146 Hamburg, Germany. c Biognos AB, Generatorsgatan 1, Box 8963, 40274 Göteborg, Sweden. d Deutsches Rheuma-Forschungszentrum, Charitéplatz 1, 10117 Berlin, Germany. E-mail: [email protected] Dedicated to Professor Rainer Beckert on the occasion of his 60th birthday Abstract A potential monosaccharidic mimetic of the sialyl LewisX tetrasaccharide (sLeX) was identified based on an in silico docking study using the crystal structure of an E-selectin-sLeX complex. The chemical synthesis of the mimetic in an ortho-selective C-glycosylation is described. This compound and two close analogues were evaluated in a cell-based selectin binding assay where none of the tested mimetics showed an IC50 below 1mM. This result can be explained by an 1 unexpected C4 conformation of the mannosyl residue which precludes the required binding of the Ca2+-ion in E-selectin. Keywords: Carbohydrates, phenols, C-glycosides, cell adhesion, molecular modelling Introduction Oligosaccharides are known to play an important role in cell recognition processes and in the communication between cells in higher organisms.1 A prominent example is the recruitment of leucocytes during the inflammatory cascade, which is initiated by the interaction between selectins and their cognate oligosaccharide ligands such as sialyl LewisX (sLeX, 1) (Fig.1).2 Undesired interactions caused by overexpression or dysregulation have been associated with various diseases e.g. -

19 Do Not Duplicate Functional Oligosaccharides a Trisaccharide Of
Do not duplicate Introduction to Carbohydrates: Oligosaccharides Dr. Yuan Yao Whistler Center for Carbohydrate Research Short Course October 3, 2017 Basic Concepts 2 Do not duplicate • “Oligo-” is the prefix from Greek language “few”; Poly- “many” • Oligosaccharides: Products of glycosidic linkages of 2-20 monosaccharide units (most commonly 2-9). Polysaccharides: More than 20 units • In the disaccharides: the aglycone is a monosaccharide unit; higher order oligosaccharides are named “tri-”, “tetra-”, “penta-”, etc. • There can be α-/β-(1→2), (1→3), (1→4) or (1→6) glycosidic linkages, with different stabilities & digestibilities (as for human body) • The structures of oligosaccharides could be linear or branched. Linear: head-to-tail linkage, 1 reducing end, 1 non-reducing end Branched: 1 reducing end, multiple non-reducing ends Common Disaccharides 3 Do not duplicate • Disaccharides are the simplest oligosaccharides that are only composed of two monosaccharide units o Highly abundant in nature; or the products of incomplete hydrolysis of higher oligosaccharides or polysaccharides o Water-soluble, with sweet taste • Most Common: Sucrose, Maltose, Lactose, & Trehalose o Naturally occurring o As the main product of photosynthesis, sucrose is ubiquitous in all plants, with high abundance in sugar cane and beet, as well as fruits o Commonly known as table sugar, sucrose usually serves as a “standard” of sweetness for other sweeteners Common Disaccharides 4 Do not duplicate Sucrose • A disaccharide of one glucose and one fructose unit, connected via β-(1,2)-glycosidic linkage • The “head-to-head” linkage is unstable due to high strain, and is therefore easily hydrolyzed (acid-catalyzed, or enzymatic) • Sucrose is a non-reducing sugar α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside 5 Common Disaccharides Inverted sugarDo (syrup) not duplicate • Sucrose could be readily hydrolyzed. -

Chapter 9 PRODUCTION and BIOACTIVITY OF
Chapter 9 PRODUCTION AND BIOACTIVITY OF OLIGOSACCHARIDES DERIVED FROM LACTOSE Villamiel, M., Montilla, A.*, Olano, A. & Corzo, N. Instituto de Investigación en Ciencias de la Investigación CIAL (CSIC-UAM), C/ Nicolás Cabrera, 9. Campus de la Universidad Autónoma de Madrid 28049 Madrid, Spain * Corresponding author: Instituto de Investigación en Ciencias de la Alimentación, CIAL (CSIC-UAM). CEI (UAM+CSIC), Nicolás Cabrera, 9, E-28049, Madrid, Spain. E-mail: [email protected] Tel +34 910017952; Fax +34 910017905 1 Abstract 9.1. Introduction 9.2. Mono- and disaccharides 9.2.1. Tagatose 9.2.1.1. Chemical isomerisation 9.2.1.2. Enzymatic synthesis 9.2.1.3. Uses of tagatose 9.2.2. Lactulose 9.2.2.1. Isomerisation of lactose 9.2.2.2. Transgalactosylation of lactose 9.2.2.3. Uses of lactulose 9.2.3. Epilactose 9.3. Lactosucrose 9.3.1. Enzymatic transfructosylation of lactose 9.3.2. Enzymatic transgalactosylation of sucrose 9.3.3. Uses of lactosucrose 9.4. Galactooligosaccharides 9.4.1. Enzymatic synthesis from lactose 9.4.2. Enzymatic synthesis from lactulose 9.4.3. Chemical isomerisation 9.4.4. Assessment of beneficial effects of oligosaccharides derived from lactulose 9.4.5. Uses of galactooligosaccharides 9.5. Other oligosaccharides 9.6. Purification of carbohydrates derived from lactose 2 Abstract In an attempt to offer a broad overview on lactose applications as a source of bioactive carbohydrates, in this chapter the authors have collected the most recent investigations on methods to produce these compounds, which have a positive effect on gut microbiota. Chemical and biological productions of tagatose, lactulose, lactosucrose, lactulosucrose and galactooligosaccharides, among others, have been described. -

Southern York County School District Instructional Plan
Southern York County School District Instructional Plan Name: Dates: September Course/Subject: Organic Chemistry and Unit Plan 1: Organic Chemistry Biochemistry Stage 1 – Desired Results PA Standard(s)/Assessment Anchors Addressed: 3.4.12A—Apply rules of systematic nomenclature and formula writing to chemical substances 3.4.12A—Classify and describe, in equation form, types of chemical and nuclear reactions 3.4.12A—Characterize and identify important classes of compounds 3.4.10A—Understand that carbon can form several different types of compounds. 3.2.12B—Evaluate experimental information for appropriateness and adherence to relevant science processes. Understanding(s): Essential Question(s): Students will understand . 1. The structure and function of organic . Why do we study organic chemistry? compounds affects our everyday life. Learning Objectives: Students will know . Students will be able to: . Organic chemistry is the study of . Support why organic chemistry is a compounds containing carbon. separate branch of chemistry. Chemists obtain organic compounds . Provide examples of organic either by isolation from plant and animal compounds that are isolated from sources or by synthesis in the nature. laboratory. Provide examples of organic . Carbon normally forms four bonds and compounds that are synthesized in the has no unshared pairs of electrons. laboratory. Nitrogen normally forms three bonds . Compare and contrast molecular and has one unshared pair of electrons. formulas and structural formulas. Oxygen normally forms two bonds and . Draw structural formulas from given has two unshared pairs of electrons. molecular formulas using the . A functional group is a site of HONC1234 rule. chemical reactivity; a particular . Identify functional groups in structural functional group, in whatever compound formulas. -

Recent Advances in Synthesis of Bacterial Rare Sugar Building Blocks and Their Applications
Natural Product Reports Recent Advances in Synthesis of Bacterial Rare Sugar Building Blocks and Their Applications Journal: Natural Product Reports Manuscript ID: NP-HIG-01-2014-000003.R1 Article Type: Highlight Date Submitted by the Author: 13-Feb-2014 Complete List of Authors: Emmadi, Madhu; IIT Bombay, Department of Chemistry; Kulkarni, Suvarn; IIT Bombay, Deapartment of Chemistry Page 1 of 11 Natural Product Reports NPR RSCPublishing HIGHLIGHT Recent Advances in Synthesis of Bacterial Rare Sugar Building Blocks and Their Applications Cite this: DOI: 10.1039/x0xx00000x Madhu Emmadi and Suvarn S. Kulkarni* Received 00th January 2012, Covering: 1964 to 2013 Accepted 00th January 2012 DOI: 10.1039/x0xx00000x Bacteria have unusual glycans on their surfaces which distinguish them from the host cells. These www.rsc.org/ unique structures offer avenues for targeting bacteria with specific therapeutics and vaccines. However, the rare sugars are not accessible in acceptable purity and amounts by isolation from natural sources. Thus, procurement of orthogonally protected rare sugar building blocks through efficient chemical synthesis is regarded as a crucial step towards the development of glycoconjugate vaccines. This Highlight focuses on recent advances in the synthesis of the bacterial deoxy amino hexopyranoside building blocks and their applications to construct various biologically important bacterial O‐glycans. 1. Introduction Bacterial glycoproteins and oligosaccharides contain rare deoxy 1- amino sugars which are not present on the human cell surface. 6 6 X 4 These important structural differences help to differentiate 5 O between the pathogen and the host cell and can be exploited for HO 3 2 1 OH target specific drug discovery and carbohydrate based vaccine AcHN development.7 However, these rare sugars are not available from natural sources.