Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of Total Dietary Fibre and Available Carbohydrates: a Rapid Integrated Procedure That Simulates in Vivo Digestion
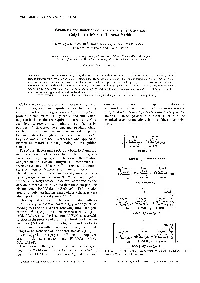
860 DOI 10.1002/star.201500017 Starch/Stärke 2015, 67, 860–883 RESEARCH ARTICLE Determination of total dietary fibre and available carbohydrates: A rapid integrated procedure that simulates in vivo digestion Barry V. McCleary, Naomi Sloane and Anna Draga Megazyme International Ireland, Bray Business Park, Bray, County Wicklow, Ireland The new definition of dietary fibre introduced by Codex Alimentarius in 2008 includes resistant Received: January 23, 2015 starch and the option to include non-digestible oligosaccharides. Implementation of this definition Revised: March 5, 2015 required new methodology. An integrated total dietary fibre method was evaluated and accepted by Accepted: March 5, 2015 AOAC InternationalandAACCInternational(AOACMethods2009.01and2011.25;AACCMethod 32–45.01 and 32–50.01, and recently adopted by Codex Alimentarius as a Type I Method. However, in application of the method to a diverse range of food samples and particularly food ingredients, some limitations have been identified. One of the ongoing criticisms of this method was that the time of incubation with pancreatic a-amylase/amyloglucosidase mixture was 16 h, whereas the time for food to transit through the human small intestine was likely to be approximately 4 h. In the current work, we use an incubation time of 4 h, and have evaluated incubation conditions that yield resistant starch and dietary values in line with ileostomy results within this time frame. Problems associated with production, hydrolysis and chromatography of various oligosaccharides have been addressed resulting in a more rapid procedure that is directly applicable to all foods and food ingredients currently available. Keywords: Available carbohydrates / Codex Alimentarius / Dietary fibre determination / Enzymic / Non-digestible oligosaccharides / Resistant starch : Additional supporting information may be found in the online version of this article at the publisher’s web-site. -
Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(Glcnach Tetrasaccharide
Polymer Journal, Vol. 30. No. 8, pp 653-658 ( 1998) Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(GlcNAch Tetrasaccharide Kazukiyo KoBAYASHI,t Shoko KAMIYA, Minoru MATSUYAMA,* Takeomi MURATA,* and Taichi Usur* Graduate School of' Engineering, Nagoya University, Chikusa, Nagoya 464-8603, Japan * Faculty of' Agriculture, Shizuoka University, Ohya, Shizuoka 422---8529, Japan (Received February 16, 1998) ABSTRACT: Tetrasaccharide Galfil--+4(GlcNAch was synthesized from N,N',N"-triacetylchitotriose (GlcNAc)s and lactose using transglycosylation with a P-o-galactosidase from Bacillus circu/ans. The reducing terminal of Galfil--+4(GlcNAclJ was oxidized and connected to p-vinylbenzylamine via amide linkage, and the resulting oligosaccharide-substituted styrene monomer was polymerized with the radical initiator, 2,2' -azobis(2-amidinopropane) dihydrochloride at 60"C. Glycopolystyrene was found to bind strongly with wheat germ agglutinin (WGA) and tomato (Lycopersicon esculentum) agglutinin (LEA) by inhibition of hemagglutination and double diffusion. KEY WORDS Oligosaccharides / Glycopolymers / Lectins / Enzymatic Synthesis/ Recognition / Cell surface carbohydrates from glycoproteins, glyco dimensional immunodiffusion in agar and inhibition of lipids, proteoglycans, and capsular polysaccharides play lectin-induced hemagglutination. Comparison was made important roles in biological events, 1.2 Carbohydrate using analogous homopolymers carrying N-acetyllactos protein interaction is usually weak and multivalent amine and chitooligosaccharides. Chart I illustrates the oligosaccharide chains are required to target cell surface chemical structures and abbreviations of these glycopoly carbohydrate receptors and inhibit host infection by mers. pathogens. 3 Glycopolymers carrying pendant oligo saccharide chains can be regarded as multivalent ligands known to interact strongly with lectins and antibodies, 4 · 5 Glycopolymers have been synthesized and applied as biomedical materials having biological recognition signals. -

A Versatile Glycosylation Strategy Via Au(III) Catalyzed Activation of Thioglycoside Donors† Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue A versatile glycosylation strategy via Au(III) catalyzed activation of thioglycoside donors† Cite this: Chem. Sci.,2016,7,4259 Amol M. Vibhute, Arun Dhaka, Vignesh Athiyarath and Kana M. Sureshan* Among various methods of chemical glycosylations, glycosylation by activation of thioglycoside donors using a thiophilic promoter is an important strategy. Many promoters have been developed for the activation of thioglycosides. However, incompatibility with substrates having alkenes and the requirement of a stoichiometric amount of promoters, co-promoters and extreme temperatures are some of the limitations. We have developed an efficient methodology for glycosylation via the activation of thioglycoside donors using a catalytic amount of AuCl3 and without any co-promoter. The reaction is Received 10th February 2016 very fast, high-yielding and very facile at room temperature. The versatility of this method is evident from Accepted 4th March 2016 the facile glycosylation with both armed and disarmed donors and sterically demanding substrates DOI: 10.1039/c6sc00633g (acceptors/donors) at ambient conditions, from the stability of the common protecting groups, and from www.rsc.org/chemicalscience the compatibility of alkene-containing substrates during the reaction. Creative Commons Attribution 3.0 Unported Licence. Introduction alkenes;8 and (iv) the requirement of extremely low temperatures for the reaction. Development of novel and milder methods of Various forms of carbohydrates play important biological roles thioglycoside activation that overcome these limitations is an 5 ,6 and hence the chemical synthesis of glycoconjugates and agenda of utmost importance among chemists. g a Pohl et al. -

Tetrasaccharide Glycoforms from Bacillus Anthracis Exosporium and Fragments Thereof
molecules Article Structure-Immunogenicity Relationship of α- and β-Tetrasaccharide Glycoforms from Bacillus anthracis Exosporium and Fragments Thereof Riccardo De Ricco 1, Christy L. Ventura 2, Filippo Carboni 1, Rina Saksena 3,†, Pavol Kováˇc 3 and Roberto Adamo 1,* ID 1 GSK, Research Centre, via Fiorentina 1, 53100 Siena, Italy; [email protected] (R.D.R.); fi[email protected] (F.C.) 2 Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; [email protected] 3 NIDDK, LBC, National Institutes of Health, Bethesda, MD 20892-0815, USA; [email protected] (R.S.); [email protected] (P.K.) * Correspondence: [email protected]; Tel.: +39-0577-539393 † Current address: Department of Chemistry, The University of New Mexico, Albuquerque, NM 87131, USA. Received: 12 July 2018; Accepted: 17 August 2018; Published: 20 August 2018 Abstract: The tetrasaccharide (2-O-methyl-4-(3-hydroxy-3-methylbutamido)-4,6-dideoxy-α-D- glucopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!2)-L-rhamnopyranose) from the major exosporium protein (BclA) of Bacillus anthracis has been proposed as a target for development of diagnostics and immune therapy or prophylaxis. While the immunodominant character of the anthrose residue has been previously elucidated, the role of the stereochemical configuration of the downstream rhamnose is unknown. Because the linkage of this residue to the GlcNAc bridging the glycan and the protein is lost during isolation of the tetrasaccharide, its α- and β-glycoforms have been synthesized. Herein, we prepared neoglycoconjugates from a series of fragments of the tetrasaccharide, including the complete α- and β-tetrasaccharide glycoforms, a 2-demethoxylated version of the α-tetrasaccharide, and the α- and β-trirhamnosides and CRM197. -

Quantitative Changes in Various Sugar Concentrations During Fermentation
QUAriTri'AT:CVE GHAI'jGES 111 VARIOUS SUGAR GOIjCm'RATlOKS UJRIMG FEH'IiSm'ATlOII Oi<' DOUGliS, SPONGiiS AMD BRliT/ZS ^' Ting Jui Tan^ 3. S., Tai-.v-an Provincial ChTJungshing Universitj . A MSTEK'S THESIS subnitted in partial fulfilLTient of the req-uireiaent Tor the decree MSTitlR OF SCIENCE Food Science Depairtinsnt Ox Grain Science arid Ladiistiy KAIIS'IS STATK UNXVilRSI'i'Y Manhattan, Kansas 1963 ApTrov'i'id by: I'lajor Professor . ' - LD . /?(? . tSX table of coiitehts eitroduction 1 REVIEV7 OF LITER/iTUPJi; 2 I. The Carbohydrates of Ivlieat and VJheat Flour 2 II. Quantitative Determination of Sugars in Natural ............ Materials 5 III, The Fate of Sugars in Breadmaking ......... 11 ^lETHOD AND MATERIALS l6 I. Flour Samples l6 II. Formulation, Dough I-Iake-up and Balcing 16 III, Determination of Gas Production Rates 19 IV. Schedule of Sample Collection for Sugar Analyses ..... 20 V. Method of Ejctraction of Sugars from Dough, Brew, Sponge and Bread Crumb . , 21 VI. tiethods of Analyses , 21 RESULTS AND DISCUSSION 25 I. of Discussion the Experimental Methods , 25 II. Change in Total Reducing Sugars of the Extracts 31 III. Change of Concentration of Individual Sugars During Fermentation ........... ....... 32 IV. Rate of Gas Production 34 V. Residual Sugars in Bread 36 SU>2^SIRY AND CONCLUSION 37 ACKI^Oi-niEDGEMEIT Zfo LITERATURE CITED , ^ i^l ii LIST _0F TABLES I. The I-Iono- and Di- saccharide Content of Flour , 3 II, Changes of Sugar Content in Steeped and Germinated Ivheat , , , , 12 III, Size of Sar.ples Used in Detenrdnation of Gas Production Hates , . 19 IV. -

Effect of Glycosylation on Protein Folding: a Close Look at Thermodynamic Stabilization
Effect of glycosylation on protein folding: A close look at thermodynamic stabilization Dalit Shental-Bechor and Yaakov Levy* Department of Structural Biology, Weizmann Institute of Science, Rehovot 76100, Israel Edited by Jose´N. Onuchic, University of California at San Diego, La Jolla, CA, and approved May 1, 2008 (received for review February 10, 2008) Glycosylation is one of the most common posttranslational mod- ical functioning of proteins in the cell. Understanding the effects ifications to occur in protein biosynthesis, yet its effect on the of posttranslational modifications to the protein energy land- thermodynamics and kinetics of proteins is poorly understood. A scape is valuable in understanding protein function and how minimalist model based on the native protein topology, in which protein thermodynamics and kinetics can be modulated by the each amino acid and sugar ring was represented by a single bead, formation of a conjugate or through an external stimulus. In this was used to study the effect of glycosylation on protein folding. article, we explore the effects of glycosylation on the biophysical We studied in silico the folding of 63 engineered SH3 domain properties of proteins with the main goal of understanding variants that had been glycosylated with different numbers of folding mechanisms, thermodynamics, and kinetics in the conjugated polysaccharide chains at different sites on the protein’s context of the cell. surface. Thermal stabilization of the protein by the polysaccharide Glycosylation [i.e., the attachment of polysaccharide chains chains was observed in proportion to the number of attached (also termed ‘‘glycans’’) to proteins] is regarded as one of the chains. -

Nutritive Sweeteners from Corn Have Become America’S Premier Sweeteners
NutritiveNutritive SweetenersSweeteners FromFrom CornCorn CONTENTS Member Companies and Plant Locations ....................................... 2 Foreword .......................................................................................... 3 Historical Perspective ...................................................................... 4 Research and development orientation ....................................... 5 Technology aimed at needs .......................................................... 7 Growth, Development and Diversity ............................................. 7 CONTENTS Classification and Nutrition ............................................................ 9 Classification ................................................................................. 9 Corn sweeteners in nutrition ..................................................... 10 Technical Background ................................................................... 11 Corn starch ................................................................................. 11 Starch hydrolysis ........................................................................ 13 Crystalline dextrose .................................................................... 14 Dextrose isomerization .............................................................. 15 Manufacture ................................................................................... 17 Corn syrups ................................................................................ 17 Dried corn syrups ...................................................................... -

A Thesis Entitled Thio-Arylglycosides with Various Aglycon Para-Substituents, a Useful Tool for Mechanistic Investigation Of
A Thesis entitled Thio-arylglycosides with Various Aglycon Para-Substituents, a Useful Tool for Mechanistic Investigation of Chemical Glycosylations by Xiaoning Li Submitted as partial fulfillment of the requirements for the Master of Science Degree in Chemistry ___________________________ Advisor: Dr. Xuefei Huang ___________________________ College of Graduate Studies The University of Toledo August 2007 An Abstract of Thio-arylglycosides with Various Aglycon Para-Substituents, a Useful Tool for Mechanistic Investigation of Chemical Glycosylations by Xiaoning Li Submitted as partial fulfillment of the requirements for the Master of Science Degree in Chemistry The University of Toledo August 2007 Oligosaccharides are usually found as protein or lipid conjugates in cellular systems. They play crucial roles in many biological processes. Among many approaches, organic synthesis is a very important way to obtain the desired oligosaccharides for biological studies. To date, no general synthetic procedures are available for oligosaccharide synthesis. Laborious synthetic transformations are generally required in order to obtain the desired regio- and/or stereo-selective control in oligosaccharide synthesis, due to their diverse and complex structures and many chemical equivalent ii hydroxyl functional groups. To achieve a rapid synthetic routine with high yields, a key step - glycosylation in oligosaccharide synthesis needs to be well understood. Thus an insight into the mechanism of glycosylation will provide valuable information potentially leading to the development of generalized glycosylation method. In this work, kinetic properties of glycosylation were evaluated by model reactions between three different series of glycosyl donors and three different glycosyl acceptors. The glycosylation mechanism was analyzed in the context of a linear-free energy relationship. -

Isolation and Characterization of a Blood Group A-Specific Urinary Tetrasaccharide
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Volume 119, number 1 FEBS LETTERS September 1980 ISOLATION AND CHARACTERIZATION OF A BLOOD GROUP A-SPECIFIC URINARY TETRASACCHARIDE Christian DERAPPE+. Arne LUNDBLAD*, Lisbeth MESSETER and Sigfrid SVENSSON Department of Clinical Chemistry, University Hospital, S-221 85 Lund, Sweden Received 21 July 1980 1. Introduction also collected from a blood group A secretor without any dietary restrictions. All urines were stored at Blood group A-, B- and H-active tetra- en penta- -20°C until required. The 6 h urine portions were saccharides have been isolated from urine of starved deionized by passage through a mixed-bed ion- ABH secretors [ 1,2]. Ingestion of free galactose or a exchange resin. To 20 ml of the desalted urine 200 pg glycoside of galactose (lactose) induces the formation isomaltotetraose were added as internal standard and and excretion of blood group specific di- and tri- the sample was reduced and permethylated. The saccharides [3,4] and small amounts of hexa- and permethylated oligosaccharides were analyzed by heptasaccharides [4]. The serological activities of GLC-MS [6]. some A- and B-specific oligosaccharides have been Isolation of the A-active tetrasaccharide was car- studied and compared with those of oligosaccharides ried out using gel chromatography on Sephadex G-l 5 from soluble blood group A and B substances [3,5]. (for experimental details see legend to fig.l), and When urine from individuals of different ABO blood preparative paper chromatography was done on group and secretor status was analyzed by gas-liquid Whatman no. -

Characterization of Glycosyl Dioxolenium Ions and Their Role in Glycosylation Reactions
ARTICLE https://doi.org/10.1038/s41467-020-16362-x OPEN Characterization of glycosyl dioxolenium ions and their role in glycosylation reactions Thomas Hansen 1,4, Hidde Elferink2,4, Jacob M. A. van Hengst1, Kas J. Houthuijs 2, Wouter A. Remmerswaal 1, Alexandra Kromm2, Giel Berden 3, Stefan van der Vorm 1, Anouk M. Rijs 3, Hermen S. Overkleeft1, Dmitri V. Filippov1, Floris P. J. T. Rutjes2, Gijsbert A. van der Marel1, Jonathan Martens3, ✉ ✉ ✉ Jos Oomens 3 , Jeroen D. C. Codée 1 & Thomas J. Boltje 2 1234567890():,; Controlling the chemical glycosylation reaction remains the major challenge in the synthesis of oligosaccharides. Though 1,2-trans glycosidic linkages can be installed using neighboring group participation, the construction of 1,2-cis linkages is difficult and has no general solution. Long-range participation (LRP) by distal acyl groups may steer the stereoselectivity, but contradictory results have been reported on the role and strength of this stereoelectronic effect. It has been exceedingly difficult to study the bridging dioxolenium ion intermediates because of their high reactivity and fleeting nature. Here we report an integrated approach, using infrared ion spectroscopy, DFT computations, and a systematic series of glycosylation reactions to probe these ions in detail. Our study reveals how distal acyl groups can play a decisive role in shaping the stereochemical outcome of a glycosylation reaction, and opens new avenues to exploit these species in the assembly of oligosaccharides and glycoconju- gates to fuel biological research. 1 Leiden University, Leiden Institute of Chemistry, Einsteinweg 55, 2333 CC Leiden, The Netherlands. 2 Radboud University Institute for Molecules and Materials, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands. -

Glycosyltransferases: Mechanisms and Applications in Natural Product Development
Chemical Society Reviews Glycosyltransferases: mechanisms and applications in natural product development Journal: Chemical Society Reviews Manuscript ID: CS-REV-07-2015-000600 Article Type: Review Article Date Submitted by the Author: 31-Jul-2015 Complete List of Authors: Liang, Dongmei; School of Chemical Engineering and Technology, Department of Pharmaceutical Engineering Liu, Jiaheng; School of Chemical Engineering and Technology, Department of Pharmaceutical Engineering Wu, Hao; School of Chemical Engineering and Technology, Department of Pharmaceutical Engineering Wang, Binbin; School of Chemical Engineering and Technology, Department of Pharmaceutical Engineering Zhu, Hongji; School of Chemical Engineering and Technology, Department of Pharmaceutical Engineering Qiao, Jianjun; School of Chemical Engineering and Technology, Tianjin University, Page 1 of 75 Chemical Society Reviews Glycosyltransferases: mechanisms and applications in natural product development Dong-Mei Liang ‡, Jia-Heng Liu ‡, Hao Wu, Bin-Bin Wang, Hong-Ji Zhu and Jian-Jun Qiao ∗ Department of Pharmaceutical Engineering, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China; Key Laboratory of Systems Bioengineering, Ministry of Education Tianjin 300072, China; SynBio Research Platform, Collaborative Innovation Center of Chemical Science and Engineering , Tianjin 300072, China. ‡ Dong-Mei Liang and Jia-Heng Liu contributed equally to this work. ∗ Corresponding author. Tel: +86-022-8740-2107; Fax: +86-022-8740-2107. E-mail address: [email protected] Chemical Society Reviews Page 2 of 75 Abstract Glycosylation reactions mainly catalyzed by glycosyltransferases (Gts), occur almost everywhere in biosphere, and always play crucial roles in vital processes. In order to understand the full potential of Gts, the chemical and structural glycosylation mechanisms are systematically summarized in this review, including some new outlooks in inverting/retaining mechanisms and the overview of GT-C superfamily proteins as a novel Gt fold. -

Fluoride Migration Catalysis Enables Simple, Stereoselective, and Iterative Glycosylation Girish C
Fluoride Migration Catalysis Enables Simple, Stereoselective, and Iterative Glycosylation Girish C. Sati, Joshua L. Martin, Yishu Xu, Tanmay Malakar, Paul M. Zimmerman, and John Montgomery* Department of Chemistry, University of Michigan, Ann Arbor, MI 48109-1055, USA *Corresponding author. Email: [email protected] Abstract: Challenges in the assembly of glycosidic bonds pose a bottleneck in enabling the remarkable promise of advances in the glycosciences. We report a strategy that applies unique features of electrophilic boron catalysts in addressing current limitations of methods in glycoside synthesis. The strategy utilizes glycosyl fluoride donors and silyl ether acceptors while tolerating the Lewis basic environment found in carbohydrates. The method allows a simple setup at room temperature while utilizing catalyst loadings as low as 0.5 mol %, and air- and moisture stable forms of the catalyst are found to be effective. These characteristics enable a wide array of glycosylation patterns to be accessed, including all four C1-C2 stereorelationships, and the method allows one-pot, iterative glycosylations to generate oligosaccharides directly from monosaccharide building blocks. Main Text: Advances in the glycosciences present enormous promise in the understanding of disease and the development of strategies for improving human health.(1-3) The structural diversity and resulting biological properties of oligosaccharides and glycoconjugates are amplified by the stereochemical variations present within monosaccharide building blocks, the positioning (or absence) of oxygen and nitrogen functionality, and the multitude of possible points of connection between monosaccharide units. These features, paired with the multivalency of binding interactions, provide oligosaccharide chains with exquisite properties that govern many molecular recognition events in biology.