Chapter 9 PRODUCTION and BIOACTIVITY OF
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of Total Dietary Fibre and Available Carbohydrates: a Rapid Integrated Procedure That Simulates in Vivo Digestion
860 DOI 10.1002/star.201500017 Starch/Stärke 2015, 67, 860–883 RESEARCH ARTICLE Determination of total dietary fibre and available carbohydrates: A rapid integrated procedure that simulates in vivo digestion Barry V. McCleary, Naomi Sloane and Anna Draga Megazyme International Ireland, Bray Business Park, Bray, County Wicklow, Ireland The new definition of dietary fibre introduced by Codex Alimentarius in 2008 includes resistant Received: January 23, 2015 starch and the option to include non-digestible oligosaccharides. Implementation of this definition Revised: March 5, 2015 required new methodology. An integrated total dietary fibre method was evaluated and accepted by Accepted: March 5, 2015 AOAC InternationalandAACCInternational(AOACMethods2009.01and2011.25;AACCMethod 32–45.01 and 32–50.01, and recently adopted by Codex Alimentarius as a Type I Method. However, in application of the method to a diverse range of food samples and particularly food ingredients, some limitations have been identified. One of the ongoing criticisms of this method was that the time of incubation with pancreatic a-amylase/amyloglucosidase mixture was 16 h, whereas the time for food to transit through the human small intestine was likely to be approximately 4 h. In the current work, we use an incubation time of 4 h, and have evaluated incubation conditions that yield resistant starch and dietary values in line with ileostomy results within this time frame. Problems associated with production, hydrolysis and chromatography of various oligosaccharides have been addressed resulting in a more rapid procedure that is directly applicable to all foods and food ingredients currently available. Keywords: Available carbohydrates / Codex Alimentarius / Dietary fibre determination / Enzymic / Non-digestible oligosaccharides / Resistant starch : Additional supporting information may be found in the online version of this article at the publisher’s web-site. -
Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(Glcnach Tetrasaccharide
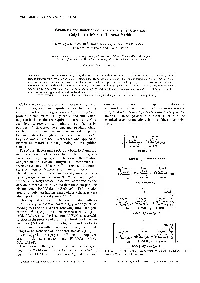
Polymer Journal, Vol. 30. No. 8, pp 653-658 ( 1998) Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(GlcNAch Tetrasaccharide Kazukiyo KoBAYASHI,t Shoko KAMIYA, Minoru MATSUYAMA,* Takeomi MURATA,* and Taichi Usur* Graduate School of' Engineering, Nagoya University, Chikusa, Nagoya 464-8603, Japan * Faculty of' Agriculture, Shizuoka University, Ohya, Shizuoka 422---8529, Japan (Received February 16, 1998) ABSTRACT: Tetrasaccharide Galfil--+4(GlcNAch was synthesized from N,N',N"-triacetylchitotriose (GlcNAc)s and lactose using transglycosylation with a P-o-galactosidase from Bacillus circu/ans. The reducing terminal of Galfil--+4(GlcNAclJ was oxidized and connected to p-vinylbenzylamine via amide linkage, and the resulting oligosaccharide-substituted styrene monomer was polymerized with the radical initiator, 2,2' -azobis(2-amidinopropane) dihydrochloride at 60"C. Glycopolystyrene was found to bind strongly with wheat germ agglutinin (WGA) and tomato (Lycopersicon esculentum) agglutinin (LEA) by inhibition of hemagglutination and double diffusion. KEY WORDS Oligosaccharides / Glycopolymers / Lectins / Enzymatic Synthesis/ Recognition / Cell surface carbohydrates from glycoproteins, glyco dimensional immunodiffusion in agar and inhibition of lipids, proteoglycans, and capsular polysaccharides play lectin-induced hemagglutination. Comparison was made important roles in biological events, 1.2 Carbohydrate using analogous homopolymers carrying N-acetyllactos protein interaction is usually weak and multivalent amine and chitooligosaccharides. Chart I illustrates the oligosaccharide chains are required to target cell surface chemical structures and abbreviations of these glycopoly carbohydrate receptors and inhibit host infection by mers. pathogens. 3 Glycopolymers carrying pendant oligo saccharide chains can be regarded as multivalent ligands known to interact strongly with lectins and antibodies, 4 · 5 Glycopolymers have been synthesized and applied as biomedical materials having biological recognition signals. -

The Food Lawyers® Respectfully Request That FDA Implements the Following
December 7, 2020 Dockets Management Staff (HFA-305) Filed Electronically Food and Drug Administration https://www.regulations.gov Re: Sugars Metabolized Differently than Traditional Sugars (FDA-2020-N-1359) Ladies and Gentlemen: One Page Executive Summary FDA’s seeks information to “… promote the public health and help consumers make informed dietary decisions” regarding sugars that are metabolized differently than traditional sugars. Given the nation’s battles with diabetes and obesity, and the benefits that non-traditional sugars can offer in these battles, the Agency’s stated public policy goal goes to the very heart of American consumers’ health. This laudatory public policy’s realization is complicated by a lack of consumer awareness of how some sugars are metabolized differently than others. In an effort to answer the questions posed by the Agency regarding the treatment of Sugars that Are Metabolized Differently Than Traditional Sugars, we suggest that the Agency adapt a mechanism that will seek to harmonize the public policy of promoting public health with consumers’ lack of awareness of sugars that are metabolized differently than sucrose. In particular, we suggest that FDA should consider the following: 1. Establish a new category of sugars called Rare Sugars that exhibit the following characteristics: a. Are naturally occurring b. Impart a sweet taste that is at least 50% the sweetness of sucrose c. 2.0 kcal/g or less. d. Resulting pH of 6.0 or greater of dental plaque after consumption. e. No or low glycemic response. f. No or low insulinemic response. 2. Exclude Rare Sugars from “Total Sugars” and “Added Sugars” declarations to stimulate their deployment by industry and consumption by the public. -

Canadian Diabetes Association National Nutrition Committee Technical Review: Non-Nutritive Intense Sweeteners in Diabetes Management
non-nutritive intense sweetener use 385 Canadian Diabetes Association National Nutrition Committee Technical Review: Non-nutritive Intense Sweeteners in Diabetes Management Réjeanne Gougeon1 PhD, Mark Spidel2 MSc, Kristy Lee3 BSc, Catherine J. Field3 PhD 1McGill Nutrition and Food Science Centre, Montreal, Quebec, Canada 2Health and Social Services, Montague, Prince Edward Island, Canada 3Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada ABSTRACT RÉSUMÉ The current Canadian Diabetes Association Clinical Practice Selon les Lignes directrices de pratique clinique actuelles de Guidelines for the Prevention and Management of Diabetes l’Association canadienne du diabète, jusqu’à 10 % des calo- in Canada state that up to 10% of daily calories can be ries consommées chaque jour peuvent provenir des sucres. derived from sugars. However, individuals with diabetes may Cependant, les personnes atteintes de diabète peuvent aussi also be relying on alternative, low-calorie sweetening agents se servir de succédanés contenant peu ou pas de calories et (providing little or no calories along with sweet taste) to con- ayant le goût du sucre pour limiter leur consommation de trol carbohydrate intake, blood glucose, weight and dental glucides, maîtriser leur glycémie et leur poids et éviter les health. Most low-calorie sweeteners, sometimes called caries dentaires. La plupart des édulcorants à faible teneur en intense or artificial sweeteners, are classified and regulated as calories, parfois appelés édulcorants de synthèse ou édulco- food additives with set acceptable daily intake (ADI) levels. rants artificiels, sont considérés comme des additifs alimen- The Health Canada Health Products and Food Branch taires et leur apport quotidien est par conséquent réglementé. -

Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments
A Dissertation Titled: Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments from B. fragilis ATCC 25285/NCTC 9343 with Alternating Charges on Adjacent Monosaccharides by Pradheep Eradi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry ___________________________________________ Dr. Peter R. Andreana, PhD, Committee Chair ___________________________________________ Dr. Steve Sucheck, PhD, Committee Member ___________________________________________ Dr. Jianglong Zhu, PhD, Committee Member ___________________________________________ Dr. Amanda C. Bryant-Freidrich, PhD, Committee Member ___________________________________________ Dr. Cyndee Gruden, Dean College of Graduate Studies The University of Toledo May 2019 Copyright 2019 Pradheep Eradi This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments from B. fragilis ATCC 25285/NCTC 9343 with Alternating Charges on Adjacent Monosaccharides by Pradheep Eradi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry The University of Toledo May 2019 Zwitterionic polysaccharides (ZPSs) are a relatively new class of carbohydrate antigens, with a paradigm shifting property; they can activate CD4+ T-cells in the absence of lipids, peptide(s) or protein(s) upon MHC class II presentation. Up until now, various anaerobic bacteria are known to express ZPSs, for example, PS A1, PS A2 and PS B (Bacteroides fragilis), Sp1 (Streptococcus pneumoniae), CP5 and CP8 (Staphylococcus aureus) and O-chain antigen (Morganella morgani). Among all the afore mentioned ZPSs, Sp1 and PS A1 polysaccharides were the prime focus of research for the past few decades and their biological properties are very well-understood. -

Tetrasaccharide Glycoforms from Bacillus Anthracis Exosporium and Fragments Thereof
molecules Article Structure-Immunogenicity Relationship of α- and β-Tetrasaccharide Glycoforms from Bacillus anthracis Exosporium and Fragments Thereof Riccardo De Ricco 1, Christy L. Ventura 2, Filippo Carboni 1, Rina Saksena 3,†, Pavol Kováˇc 3 and Roberto Adamo 1,* ID 1 GSK, Research Centre, via Fiorentina 1, 53100 Siena, Italy; [email protected] (R.D.R.); fi[email protected] (F.C.) 2 Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; [email protected] 3 NIDDK, LBC, National Institutes of Health, Bethesda, MD 20892-0815, USA; [email protected] (R.S.); [email protected] (P.K.) * Correspondence: [email protected]; Tel.: +39-0577-539393 † Current address: Department of Chemistry, The University of New Mexico, Albuquerque, NM 87131, USA. Received: 12 July 2018; Accepted: 17 August 2018; Published: 20 August 2018 Abstract: The tetrasaccharide (2-O-methyl-4-(3-hydroxy-3-methylbutamido)-4,6-dideoxy-α-D- glucopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!2)-L-rhamnopyranose) from the major exosporium protein (BclA) of Bacillus anthracis has been proposed as a target for development of diagnostics and immune therapy or prophylaxis. While the immunodominant character of the anthrose residue has been previously elucidated, the role of the stereochemical configuration of the downstream rhamnose is unknown. Because the linkage of this residue to the GlcNAc bridging the glycan and the protein is lost during isolation of the tetrasaccharide, its α- and β-glycoforms have been synthesized. Herein, we prepared neoglycoconjugates from a series of fragments of the tetrasaccharide, including the complete α- and β-tetrasaccharide glycoforms, a 2-demethoxylated version of the α-tetrasaccharide, and the α- and β-trirhamnosides and CRM197. -

Quantitative Changes in Various Sugar Concentrations During Fermentation
QUAriTri'AT:CVE GHAI'jGES 111 VARIOUS SUGAR GOIjCm'RATlOKS UJRIMG FEH'IiSm'ATlOII Oi<' DOUGliS, SPONGiiS AMD BRliT/ZS ^' Ting Jui Tan^ 3. S., Tai-.v-an Provincial ChTJungshing Universitj . A MSTEK'S THESIS subnitted in partial fulfilLTient of the req-uireiaent Tor the decree MSTitlR OF SCIENCE Food Science Depairtinsnt Ox Grain Science arid Ladiistiy KAIIS'IS STATK UNXVilRSI'i'Y Manhattan, Kansas 1963 ApTrov'i'id by: I'lajor Professor . ' - LD . /?(? . tSX table of coiitehts eitroduction 1 REVIEV7 OF LITER/iTUPJi; 2 I. The Carbohydrates of Ivlieat and VJheat Flour 2 II. Quantitative Determination of Sugars in Natural ............ Materials 5 III, The Fate of Sugars in Breadmaking ......... 11 ^lETHOD AND MATERIALS l6 I. Flour Samples l6 II. Formulation, Dough I-Iake-up and Balcing 16 III, Determination of Gas Production Rates 19 IV. Schedule of Sample Collection for Sugar Analyses ..... 20 V. Method of Ejctraction of Sugars from Dough, Brew, Sponge and Bread Crumb . , 21 VI. tiethods of Analyses , 21 RESULTS AND DISCUSSION 25 I. of Discussion the Experimental Methods , 25 II. Change in Total Reducing Sugars of the Extracts 31 III. Change of Concentration of Individual Sugars During Fermentation ........... ....... 32 IV. Rate of Gas Production 34 V. Residual Sugars in Bread 36 SU>2^SIRY AND CONCLUSION 37 ACKI^Oi-niEDGEMEIT Zfo LITERATURE CITED , ^ i^l ii LIST _0F TABLES I. The I-Iono- and Di- saccharide Content of Flour , 3 II, Changes of Sugar Content in Steeped and Germinated Ivheat , , , , 12 III, Size of Sar.ples Used in Detenrdnation of Gas Production Hates , . 19 IV. -

Nutritive Sweeteners from Corn Have Become America’S Premier Sweeteners
NutritiveNutritive SweetenersSweeteners FromFrom CornCorn CONTENTS Member Companies and Plant Locations ....................................... 2 Foreword .......................................................................................... 3 Historical Perspective ...................................................................... 4 Research and development orientation ....................................... 5 Technology aimed at needs .......................................................... 7 Growth, Development and Diversity ............................................. 7 CONTENTS Classification and Nutrition ............................................................ 9 Classification ................................................................................. 9 Corn sweeteners in nutrition ..................................................... 10 Technical Background ................................................................... 11 Corn starch ................................................................................. 11 Starch hydrolysis ........................................................................ 13 Crystalline dextrose .................................................................... 14 Dextrose isomerization .............................................................. 15 Manufacture ................................................................................... 17 Corn syrups ................................................................................ 17 Dried corn syrups ...................................................................... -

Carbohydrates and Health Report (ISBN 9780117082847)
Critical Reviews in Food Science and Nutrition ISSN: 1040-8398 (Print) 1549-7852 (Online) Journal homepage: http://www.tandfonline.com/loi/bfsn20 The scientific basis for healthful carbohydrate profile Lisa M. Lamothe, Kim-Anne Lê, Rania Abou Samra, Olivier Roger, Hilary Green & Katherine Macé To cite this article: Lisa M. Lamothe, Kim-Anne Lê, Rania Abou Samra, Olivier Roger, Hilary Green & Katherine Macé (2017): The scientific basis for healthful carbohydrate profile, Critical Reviews in Food Science and Nutrition, DOI: 10.1080/10408398.2017.1392287 To link to this article: https://doi.org/10.1080/10408398.2017.1392287 © 2017 The Author(s). Published with license by Taylor & Francis Group, LLC© Lisa M. Lamothe, Kim-Anne Lê, Rania Abou Samra, Olivier Roger, Hilary Green, and Katherine Macé Published online: 30 Nov 2017. Submit your article to this journal Article views: 859 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=bfsn20 Download by: [Texas A&M University Libraries] Date: 09 January 2018, At: 10:24 CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION https://doi.org/10.1080/10408398.2017.1392287 The scientific basis for healthful carbohydrate profile Lisa M. Lamothe, Kim-Anne Le,^ Rania Abou Samra, Olivier Roger, Hilary Green, and Katherine Mace Nestle Research Center, Vers chez les Blanc, CP44, 1000 Lausanne 26, Switzerland ABSTRACT KEYWORDS Dietary guidelines indicate that complex carbohydrates should provide around half of the calories in a Dental caries; Obesity; Type 2 balanced diet, while sugars (i.e., simple carbohydrates) should be limited to no more than 5–10% of total diabetes; Cardiovascular energy intake. -

Tagatose and Milk Allergy
ALLERGY Net Tagatose and milk allergy S. L. Taylor*, D. M. Lambrecht, S. L. Hefle Key words: allergy; cows' milk; tagatose. Tagatose is a new food ingredient being used as a reduced-calorie sweetener in foods and bever- ages. Tagatose is a six-carbon ke- Tagatose does not tose sugar found contain milk residues. naturally in some dairy products and in tropical date trees. As tagatose is incompletely absorbed, it provides only 1.5 cal/g as contrary to 4 cal/g for sucrose. Tagatose has approximately the same sweetness as sucrose. Recently, a manufacturing process for tagatose has been developed allowing the production of increased quantities of tagatose. Tagatose has been affirmed as Generally Recognized as Safe (GRAS) in the US and is approved for use in foods and beverages in the US, Korea, Australia and New Zealand. Tagatose is a unique ketose sugar manufactured by the isomerization of galactose. The galactose involved in the manufacturing of tagatose is derived from lactose, the disaccharide found in the whey fraction of milk. Lactose is known to contain residual milk proteins including several of the major cowsÕ milk allergens, principally b-lactoglobulin and a-lactalbumin from whey, on occasion (1). Consequently, some questions were raised about the potential allergenicity of tagatose. Although lactose often contains resid- ual milk allergens, tagatose is much less likely to contain any milk allergens based upon knowledge of the process used to 412 ALLERGY Net produce tagatose. The steps involved in at sensitivity levels of 2.5 p.p.m. (mg/ the manufacturing of tagatose from kg) for casein and 1.0 p.p.m. -

Isolation and Characterization of a Blood Group A-Specific Urinary Tetrasaccharide
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Volume 119, number 1 FEBS LETTERS September 1980 ISOLATION AND CHARACTERIZATION OF A BLOOD GROUP A-SPECIFIC URINARY TETRASACCHARIDE Christian DERAPPE+. Arne LUNDBLAD*, Lisbeth MESSETER and Sigfrid SVENSSON Department of Clinical Chemistry, University Hospital, S-221 85 Lund, Sweden Received 21 July 1980 1. Introduction also collected from a blood group A secretor without any dietary restrictions. All urines were stored at Blood group A-, B- and H-active tetra- en penta- -20°C until required. The 6 h urine portions were saccharides have been isolated from urine of starved deionized by passage through a mixed-bed ion- ABH secretors [ 1,2]. Ingestion of free galactose or a exchange resin. To 20 ml of the desalted urine 200 pg glycoside of galactose (lactose) induces the formation isomaltotetraose were added as internal standard and and excretion of blood group specific di- and tri- the sample was reduced and permethylated. The saccharides [3,4] and small amounts of hexa- and permethylated oligosaccharides were analyzed by heptasaccharides [4]. The serological activities of GLC-MS [6]. some A- and B-specific oligosaccharides have been Isolation of the A-active tetrasaccharide was car- studied and compared with those of oligosaccharides ried out using gel chromatography on Sephadex G-l 5 from soluble blood group A and B substances [3,5]. (for experimental details see legend to fig.l), and When urine from individuals of different ABO blood preparative paper chromatography was done on group and secretor status was analyzed by gas-liquid Whatman no. -

Potent Inhibitory Effects of D-Tagatose on the Acid Production and Water-Insoluble Glucan Synthesis of Streptococcus Mutans GS5 in the Presence of Sucrose
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Okayama University Scientific Achievement Repository Acta Med. Okayama, 2015 Vol. 69, No. 2, pp. 105ン111 CopyrightⒸ 2015 by Okayama University Medical School. Original Article http ://escholarship.lib.okayama-u.ac.jp/amo/ Potent Inhibitory Effects of D-tagatose on the Acid Production and Water-insoluble Glucan Synthesis of Streptococcus mutans GS5 in the Presence of Sucrose Daijo Sawadaa,b*, Takaaki Ogawaa, Minoru Miyakea, Yoshinori Hasuib, Fuminori Yamaguchic, Ken Izumorid, and Masaaki Tokudac,d Departments of aOral and Maxillofacial Surgery and cCell Physiology, Faculty of Medicine, Kagawa University, Miki, Kagawa 761-0793, Japan, bHasui Dental Family Clinic, Kagawa 761-0704, Japan, dRare Sugar Research Center, Miki, Kagawa 761-0793, Japan We examined and compared the inhibitory effects of D-tagatose on the growth, acid production, and water-insoluble glucan synthesis of GS5, a bacterial strain of Streptococcus mutans, with those of xylitol, D-psicose, L-psicose and L-tagatose. GS5 was cultured for 12h in a medium containing 10オ (w/v) of xylitol, D-psicose, L-psicose, D-tagatose or L-tagatose, and the inhibitory effect of GS5 growth was assessed. Each sugar showed different inhibitory effects on GS5. Both D-tagatose and xylitol significantly inhibited the acid production and water-insoluble glucan synthesis of GS5 in the presence of 1オ (w/v) sucrose. However, the inhibitory effect of acid production by D-tagatose was significantly stronger than that of xylitol in presence of sucrose. Key words: Streptococcus mutans, D-tagatose, xylitol, acid production, water-insoluble glucan he definition of ʻrare sugarsʼ is monosaccha- the most extensively examined rare sugar, including T raides and their derivatives that are not com- anti-diabetes and anti-obesity effects [4-7].