Facile Enzymatic Synthesis of Ketoses and Chemoenzymatic Reporter Strategy for Probing Complex Glycans Liuqing Wen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determining the Effect of Small Doses of Fructose and Its Epimers on Glycemic Control
Determining the Effect of Small Doses of Fructose and its Epimers on Glycemic Control by Jarvis Clyde Noronha A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Nutritional Sciences University of Toronto © Copyright by Jarvis Clyde Noronha 2017 ii Determining the Effect of Small Doses of Fructose and its Epimers on Glycemic Control Jarvis Clyde Noronha Master of Science Department of Nutritional Sciences University of Toronto 2017 Abstract Given that sugars have emerged as the dominant nutrient of concern in diabetes, there is a need for the development of alternative sweeteners. To assess the role of small doses (5g, 10g) of fructose and allulose on postprandial glucose metabolism, we conducted an acute randomized controlled trial in individuals with type 2 diabetes. We found that small doses of allulose, but not fructose, modestly reduced the postprandial glycemic response to a 75g oral glucose load. To assess whether low-dose (< 50-g/day) fructose and all its epimers (allulose, tagatose and sorbose) lead to sustainable improvements in long-term glycemic control, we conducted a systematic review and meta-analysis of controlled feeding trials. The available evidence suggested that fructose and tagatose led to significant reductions in HbA1c and fasting glucose. Our findings highlight the need for long-term randomized controlled trials to confirm the viability of fructose and its epimers as alternative sweeteners. iii Table of Contents Contents Abstract ........................................................................................................................... -

Determination of Total Dietary Fibre and Available Carbohydrates: a Rapid Integrated Procedure That Simulates in Vivo Digestion
860 DOI 10.1002/star.201500017 Starch/Stärke 2015, 67, 860–883 RESEARCH ARTICLE Determination of total dietary fibre and available carbohydrates: A rapid integrated procedure that simulates in vivo digestion Barry V. McCleary, Naomi Sloane and Anna Draga Megazyme International Ireland, Bray Business Park, Bray, County Wicklow, Ireland The new definition of dietary fibre introduced by Codex Alimentarius in 2008 includes resistant Received: January 23, 2015 starch and the option to include non-digestible oligosaccharides. Implementation of this definition Revised: March 5, 2015 required new methodology. An integrated total dietary fibre method was evaluated and accepted by Accepted: March 5, 2015 AOAC InternationalandAACCInternational(AOACMethods2009.01and2011.25;AACCMethod 32–45.01 and 32–50.01, and recently adopted by Codex Alimentarius as a Type I Method. However, in application of the method to a diverse range of food samples and particularly food ingredients, some limitations have been identified. One of the ongoing criticisms of this method was that the time of incubation with pancreatic a-amylase/amyloglucosidase mixture was 16 h, whereas the time for food to transit through the human small intestine was likely to be approximately 4 h. In the current work, we use an incubation time of 4 h, and have evaluated incubation conditions that yield resistant starch and dietary values in line with ileostomy results within this time frame. Problems associated with production, hydrolysis and chromatography of various oligosaccharides have been addressed resulting in a more rapid procedure that is directly applicable to all foods and food ingredients currently available. Keywords: Available carbohydrates / Codex Alimentarius / Dietary fibre determination / Enzymic / Non-digestible oligosaccharides / Resistant starch : Additional supporting information may be found in the online version of this article at the publisher’s web-site. -

Xylose Fermentation to Ethanol by Schizosaccharomyces Pombe Clones with Xylose Isomerase Gene." Biotechnology Letters (8:4); Pp
NREL!TP-421-4944 • UC Category: 246 • DE93000067 l I Xylose Fermenta to Ethanol: A R ew '.) i I, -- , ) )I' J. D. McMillan I ' J.( .!i �/ .6' ....� .T u�.•ls:l ., �-- • National Renewable Energy Laboratory II 'J 1617 Cole Boulevard Golden, Colorado 80401-3393 A Division of Midwest Research Institute Operated for the U.S. Department of Energy under Contract No. DE-AC02-83CH10093 Prepared under task no. BF223732 January 1993 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, com pleteness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily con stitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Printed in the United States of America Available from: National Technical Information Service U.S. Department of Commerce 5285 Port Royal Road Springfield, VA22161 Price: Microfiche A01 Printed Copy A03 Codes are used for pricing all publications. The code is determined by the number of pages in the publication. Information pertaining to the pricing codes can be found in the current issue of the following publications which are generally available in most libraries: Energy Research Abstracts (ERA); Govern ment Reports Announcements and Index ( GRA and I); Scientific and Technical Abstract Reports(STAR); and publication NTIS-PR-360 available from NTIS at the above address. -

48 3-1-4. Reactions Using Mutant FSA A129S Another Mutant Enzyme
Results _ 3-1-4. Reactions using mutant FSA A129S Another mutant enzyme, A129S, which has serine at 129th residue instead of alanine (Fig.3-11a), has high potential to catalyze the reactions using DHA as a donor substrate. Virtually, the kcat/Km value for DHA is more than 12 fold of that of WT (Schürmann, 2001). This implies that larger amounts of products can be acquired with A129S than with WT, or unfavorable reactions with WT may be accomplished with the mutant enzyme. Firstly, FSA A129S was produced in DH5α with pUC18 plasmid vector and purified with same method as WT was done (Fig.3-11b, Table 3-8). This mutant enzyme was overexpressed well and a strong band corresponding to FSA was seen on SDS-PAGE gel (as 10~15% of total protein in crude extract). In addition, this mutant enzyme held the stability against high temperature. Thus, a heat treatment step was part of the purification protocol. However, protein solution obtained from hydroxyapatite column of the final purification step showed still a few extra protein bands on SDS-PAGE gel (Fig.3-11b). Although it contained those extra proteins, the purity appeared to be more than 90% on the gel and the specific activity was quite high enough to assay the catalytic ability of the enzyme for several substrates. Indeed, even crude extract showed higher specific activity than purified WT had (Table 3-8). Three reactions (scheme 3-1) were probed to assay catalytic ability of both FSA WT and A129S. Dihydroxyacetone (DHA) or hydroxyacetone (HA) were used as donor substrates, and formaldehyde or glycolaldehyde as acceptor. -

FDA Finalizes Allulose Guidance and Requests Information on Other Sugars Metabolized Differently Than Traditional Sugars
FDA Finalizes Allulose Guidance and Requests Information on Other Sugars Metabolized Differently Than Traditional Sugars October 19, 2020 Food, Drug, and Device FDA has taken two notable actions regarding the sugars declaration in the Nutrition Facts Label (NFL) and Supplement Facts Label (SFL). On Friday, the agency released a final guidance regarding the declaration of allulose, confirming that this monosaccharide need not be included in the declaration of “Total Sugars” or “Added Sugars,” though it must be included in the “Total Carbohydrates” declaration in the NFL. Today, FDA published a Federal Register notice requesting information about and comments on the nutrition labeling of other sugars that are metabolized differently than traditional sugars. We briefly summarize both documents below to help inform stakeholder comments on the notice, which are due to FDA by December 18, 2020. Allulose Final Guidance Allulose, or D-psicose, is a monosaccharide that can be used as a substitute for traditional sugar in food and beverage products. For purposes of nutrition labeling, FDA has generally 1 defined nutrients based on their chemical structure.0F Accordingly, when FDA updated its NFL and SFL regulations in 2016, the agency reiterated the definition of “Total Sugars” as the sum of 2 all free monosaccharides and disaccharides (e.g. glucose, fructose, and sucrose).1F FDA also added to these regulations a definition of “Added Sugars” – sugars added during the processing 3 of food, or packaged as such – and required their declaration in the NFL and SFL.2F Although the agency recognized that there are sugars that are metabolized differently than traditional sugars, FDA did not make a determination at that time as to whether allulose should be excluded from “Total Carbohydrate,” “Total Sugars,” or “Added Sugars” Declarations. -

Effects of D-Allulose on Glucose Tolerance and Insulin Response to A
Clinical care/Education/Nutrition Open access Original research BMJ Open Diab Res Care: first published as 10.1136/bmjdrc-2020-001939 on 26 February 2021. Downloaded from Effects of D- allulose on glucose tolerance and insulin response to a standard oral sucrose load: results of a prospective, randomized, crossover study Francesco Franchi ,1 Dmitry M Yaranov,1 Fabiana Rollini,1 Andrea Rivas,1 Jose Rivas Rios,1 Latonya Been,1 Yuma Tani,2 Masaaki Tokuda,3 Tetsuo Iida,2 Noriko Hayashi,2 Dominick J Angiolillo,1 Arshag D Mooradian1 To cite: Franchi F, Yaranov DM, ABSTRACT Rollini F, et al. Effects of D- Introduction Current dietary guidelines recommend Significance of this study allulose on glucose tolerance limiting sugar intake for the prevention of diabetes and insulin response to a mellitus (DM). Reduction in sugar intake may require sugar What is already known about this subject? standard oral sucrose load: substitutes. Among these, D- allulose is a non- calorie rare ► D- allulose is defined one of the rare sugars, results of a prospective, which has been shown in animal and clinical randomized, crossover study. monosaccharide with 70% sweetness of sucrose, which has shown anti- DM effects in Asian populations. However, studies, conducted mostly in Asian populations, BMJ Open Diab Res Care to have postprandial plasma glucose suppres- 2021;9:e001939. doi:10.1136/ there is limited data on the effects of D- allulose in other sive effects with antiobesity and antidiabetic bmjdrc-2020-001939 populations, including Westerners. Research design and methods This was a prospective, effects. copyright. randomized, double- blind, placebo- controlled, crossover What are the new findings? ► Supplemental material is study conducted in 30 subjects without DM. -

United States Patent Office
- 2,926,180 United States Patent Office Patented Feb. 23, 1960 2 cycloalkyl, etc. These substituents R and R' may also be substituted with various groupings such as carboxyl 2,926,180 groups, sulfo groups, halogen atoms, etc. Examples of CONDENSATION OF AROMATIC KETONES WITH compounds which are included within the scope of this CARBOHYDRATES AND RELATED MATER ALS 5 general formula are acetophenone, propiophenone, benzo Carl B. Linn, Riverside, Ill., assignor, by mesne assign phenone, acetomesitylene, phenylglyoxal, benzylaceto ments, to Universal Oil Products Company, Des phenone, dypnone, dibenzoylmethane, benzopinacolone, Plaines, Ill., a corporation of Delaware dimethylaminobenzophenone, acetonaphthalene, benzoyl No Drawing. Application June 18, 1957 naphthalene, acetonaphthacene, benzoylnaphthacene, ben 10 zil, benzilacetophenone, ortho-hydroxyacetophenone, para Serial No. 666,489 hydroxyacetophenone, ortho - hydroxy-para - methoxy 5 Claims. (C. 260-345.9) acetophenone, para-hydroxy-meta-methoxyacetophenone, zingerone, etc. This application is a continuation-in-part of my co Carbohydrates which are condensed with aromatic pending application Serial No. 401,068, filed December 5 ketones to form a compound selected from the group 29, 1953, now Patent No. 2,798,079. consisting of an acylaryl-desoxy-alditol and an acylaryl This invention relates to a process for interacting aro desoxy-ketitol include simple sugars, their desoxy- and matic ketones with carbohydrates and materials closely omega-carboxy derivatives, compound sugars or oligo related to carbohydrates. The process relates more par saccharides, and polysaccharides. ticularly to the condensation of simple sugars, their 20 Simple sugars include dioses, trioses, tetroses, pentoses, desoxy- and their omega-carboxy derivatives, compound hexoses, heptoses, octoses, nonoses, and decoses. Com sugars or oligosaccharides, and polysaccharides with aro pound sugars include disaccharides, trisaccharides, and matic ketones in the presence of a hydrogen fluoride tetrasaccharides. -
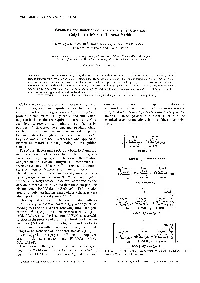
Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(Glcnach Tetrasaccharide
Polymer Journal, Vol. 30. No. 8, pp 653-658 ( 1998) Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(GlcNAch Tetrasaccharide Kazukiyo KoBAYASHI,t Shoko KAMIYA, Minoru MATSUYAMA,* Takeomi MURATA,* and Taichi Usur* Graduate School of' Engineering, Nagoya University, Chikusa, Nagoya 464-8603, Japan * Faculty of' Agriculture, Shizuoka University, Ohya, Shizuoka 422---8529, Japan (Received February 16, 1998) ABSTRACT: Tetrasaccharide Galfil--+4(GlcNAch was synthesized from N,N',N"-triacetylchitotriose (GlcNAc)s and lactose using transglycosylation with a P-o-galactosidase from Bacillus circu/ans. The reducing terminal of Galfil--+4(GlcNAclJ was oxidized and connected to p-vinylbenzylamine via amide linkage, and the resulting oligosaccharide-substituted styrene monomer was polymerized with the radical initiator, 2,2' -azobis(2-amidinopropane) dihydrochloride at 60"C. Glycopolystyrene was found to bind strongly with wheat germ agglutinin (WGA) and tomato (Lycopersicon esculentum) agglutinin (LEA) by inhibition of hemagglutination and double diffusion. KEY WORDS Oligosaccharides / Glycopolymers / Lectins / Enzymatic Synthesis/ Recognition / Cell surface carbohydrates from glycoproteins, glyco dimensional immunodiffusion in agar and inhibition of lipids, proteoglycans, and capsular polysaccharides play lectin-induced hemagglutination. Comparison was made important roles in biological events, 1.2 Carbohydrate using analogous homopolymers carrying N-acetyllactos protein interaction is usually weak and multivalent amine and chitooligosaccharides. Chart I illustrates the oligosaccharide chains are required to target cell surface chemical structures and abbreviations of these glycopoly carbohydrate receptors and inhibit host infection by mers. pathogens. 3 Glycopolymers carrying pendant oligo saccharide chains can be regarded as multivalent ligands known to interact strongly with lectins and antibodies, 4 · 5 Glycopolymers have been synthesized and applied as biomedical materials having biological recognition signals. -

Assessment of DHA in Self-Tanning Creams Applied in Spray Booths
Assessment of DHA in self-tanning creams applied in spray booths Lena Höglund MSc. DTC (Danish Toxicology Centre): Betty Bügel Mogensen Rossana Bossi Marianne Glasius National Environmental Research Institute of Denmark Survey of Chemical Substances in Consumer Products, No. 72 2006 The Danish Environmental Protection Agency will, when opportunity offers, publish reports and contributions relating to environmental research and development projects financed via the Danish EPA. Please note that publication does not signify that the contents of the reports necessarily reflect the views of the Danish EPA. The reports are, however, published because the Danish EPA finds that the studies represent a valuable contribution to the debate on environmental policy in Denmark. Contents SUMMARY AND CONCLUSIONS 5 1 INTRODUCTION 7 2 OBJECTIVES 10 3 TECHNIQUES 12 3.1 DESCRIPTION OF TECHNIQUES 12 3.1.1 Manual turbine spray 12 3.1.2 Third-generation booths (closed booths) 13 3.1.3 Fourth-generation booths (open booths) 15 3.2 SAFETY INSTRUCTIONS 16 3.2.1 General remarks on enterprises' safety instructions 16 3.2.2 Safety instructions from the authorities 16 3.2.3 Advice for customers from personnel 16 4 SUBSTANCES CONTAINED IN PRODUCTS 20 5 HEALTH ASSESSMENT 22 5.1 TOXOCOLOGICAL PROFILE OF DIHYDROXYACETONE (DHA) (CAS NO. 96-26-4) 22 5.2 BRIEF HEALTH ASSESSMENT OF ETHOXYDIGLYCOL (CAS NO. 111-90-0) 26 5.3 BRIEF HEALTH ASSESSMENT OF PHENOXYETHANOL (CAS NO.122-99-6) 27 5.4 BRIEF HEALTH ASSESSMENT OF GLYCERINE (CAS NO. 56-81-5) 27 5.5 BRIEF HEALTH ASSESSMENT OF POLYSORBATES AND SORBITAN ESTERS 27 5.6 BRIEF HEALTH ASSESSMENT OF ERYTHRULOSE (CAS NO. -

Unit 1: Carbohydrates Structure and Biological Importance: Monosaccharides, Disaccharides, Polysaccharides and Glycoconjugates
CBCS 3rd Semester Core Course VII Paper: Fundamentals of Biochemistry Unit 1: Carbohydrates Structure and Biological importance: Monosaccharides, Disaccharides, Polysaccharides and Glycoconjugates By- Dr. Luna Phukan Definition The carbohydrates are a group of naturally occurring carbonyl compounds (aldehydes or ketones) that also contain several hydroxyl groups. It may also include their derivatives which produce such compounds on hydrolysis. They are the most abundant organic molecules in nature and also referred to as “saccharides”. The carbohydrates which are soluble in water and sweet in taste are called as “sugars”. Structure of Carbohydrates Carbohydrates consist of carbon, hydrogen, and oxygen. The general empirical structure for carbohydrates is (CH2O)n. They are organic compounds organized in the form of aldehydes or ketones with multiple hydroxyl groups coming off the carbon chain. The building blocks of all carbohydrates are simple sugars called monosaccharides. A monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose) The carbohydrates can be structurally represented in any of the three forms: 1. Open chain structure. 2. Hemi-acetal structure. 3. Haworth structure. Open chain structure – It is the long straight-chain form of carbohydrates. Hemi-acetal structure – Here the 1st carbon of the glucose condenses with the -OH group of the 5th carbon to form a ring structure. Haworth structure – It is the presence of the pyranose ring structure Classification of Carbohydrates Monosaccharides The simple carbohydrates include single sugars (monosaccharides) and polymers, oligosaccharides, and polysaccharides. Simplest group of carbohydrates and often called simple sugars since they cannot be further hydrolyzed. Colorless, crystalline solid which are soluble in water and insoluble in a non-polar solvent. -

PENTOSE PHOSPHATE PATHWAY — Restricted for Students Enrolled in MCB102, UC Berkeley, Spring 2008 ONLY
Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Bryan Krantz: University of California, Berkeley MCB 102, Spring 2008, Metabolism Lecture 5 Reading: Ch. 14 of Principles of Biochemistry, “Glycolysis, Gluconeogenesis, & Pentose Phosphate Pathway.” PENTOSE PHOSPHATE PATHWAY This pathway produces ribose from glucose, and it also generates 2 NADPH. Two Phases: [1] Oxidative Phase & [2] Non-oxidative Phase + + Glucose 6-Phosphate + 2 NADP + H2O Ribose 5-Phosphate + 2 NADPH + CO2 + 2H ● What are pentoses? Why do we need them? ◦ DNA & RNA ◦ Cofactors in enzymes ● Where do we get them? Diet and from glucose (and other sugars) via the Pentose Phosphate Pathway. ● Is the Pentose Phosphate Pathway just about making ribose sugars from glucose? (1) Important for biosynthetic pathways using NADPH, and (2) a high cytosolic reducing potential from NADPH is sometimes required to advert oxidative damage by radicals, e.g., ● - ● O2 and H—O Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Two Phases of the Pentose Pathway Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY NADPH vs. NADH Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Oxidative Phase: Glucose-6-P Ribose-5-P Glucose 6-phosphate dehydrogenase. First enzymatic step in oxidative phase, converting NADP+ to NADPH. Glucose 6-phosphate + NADP+ 6-Phosphoglucono-δ-lactone + NADPH + H+ Mechanism. Oxidation reaction of C1 position. Hydride transfer to the NADP+, forming a lactone, which is an intra-molecular ester. -

Enzymatic Synthesis of L-Fucose from L-Fuculose Using a Fucose Isomerase
Kim et al. Biotechnol Biofuels (2019) 12:282 https://doi.org/10.1186/s13068-019-1619-0 Biotechnology for Biofuels RESEARCH Open Access Enzymatic synthesis of L-fucose from L-fuculose using a fucose isomerase from Raoultella sp. and the biochemical and structural analyses of the enzyme In Jung Kim1†, Do Hyoung Kim1†, Ki Hyun Nam1,2 and Kyoung Heon Kim1* Abstract Background: L-Fucose is a rare sugar with potential uses in the pharmaceutical, cosmetic, and food industries. The enzymatic approach using L-fucose isomerase, which interconverts L-fucose and L-fuculose, can be an efcient way of producing L-fucose for industrial applications. Here, we performed biochemical and structural analyses of L-fucose isomerase identifed from a novel species of Raoultella (RdFucI). Results: RdFucI exhibited higher enzymatic activity for L-fuculose than for L-fucose, and the rate for the reverse reaction of converting L-fuculose to L-fucose was higher than that for the forward reaction of converting L-fucose to L-fuculose. In the equilibrium mixture, a much higher proportion of L-fucose (~ ninefold) was achieved at 30 °C and pH 7, indicating that the enzyme-catalyzed reaction favors the formation of L-fucose from L-fuculose. When biochemical analysis was conducted using L-fuculose as the substrate, the optimal conditions for RdFucI activity were determined to be 40 °C and pH 10. However, the equilibrium composition was not afected by reaction temperature in the range 2 of 30 to 50 °C. Furthermore, RdFucI was found to be a metalloenzyme requiring Mn + as a cofactor. The comparative crystal structural analysis of RdFucI revealed the distinct conformation of α7–α8 loop of RdFucI.