Aminosugar Components and Characterization of Antibiotic K-52B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University of Cape Town
CHEMICAL AND CONFORMATIONAL STUDIES OF BACTERIAL CELL SURFACE POLYSACCHARIDE REPEATING UNITS Zaheer Timol University of Cape Town Supervisors: Neil Ravenscroft, Michelle Kuttel and David Gammon A thesis presented for the degree of Master in Science University of Cape Town March 2017 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Abstract Bacterial cell surface polysaccharides are primarily present as lipopolysaccharides or capsular polysac- charides. They are used by cells for both structure and function and have been shown to be a virulence factor of bacterial pathogens. Cell surface polysaccharides are widely utilised as antigenic components in vaccines and play an important role in the protection against numerous diseases including meningo- coccal disease and shigellosis. This study is composed of two parts: a computational section, which investigates the capsular polysaccharide (CPS) repeating unit (RU) conformations of meningococcal Y and W CPS vaccines and a second experimental component that involves synthetic studies toward the O-specific polysaccharide (O-SP) RU of Shigella sonnei. The CPS RU of MenY [!6)-a-D-Glc(1!4)-a-D-NeuNAc-(2!] and MenW [!6)-a-D-Gal(1!4)-a- D-NeuNAc-(2!] differ only in the orientation of the C-4 hydroxyl: equatorial in MenY and axial in MenW. -

Determination of Total Dietary Fibre and Available Carbohydrates: a Rapid Integrated Procedure That Simulates in Vivo Digestion
860 DOI 10.1002/star.201500017 Starch/Stärke 2015, 67, 860–883 RESEARCH ARTICLE Determination of total dietary fibre and available carbohydrates: A rapid integrated procedure that simulates in vivo digestion Barry V. McCleary, Naomi Sloane and Anna Draga Megazyme International Ireland, Bray Business Park, Bray, County Wicklow, Ireland The new definition of dietary fibre introduced by Codex Alimentarius in 2008 includes resistant Received: January 23, 2015 starch and the option to include non-digestible oligosaccharides. Implementation of this definition Revised: March 5, 2015 required new methodology. An integrated total dietary fibre method was evaluated and accepted by Accepted: March 5, 2015 AOAC InternationalandAACCInternational(AOACMethods2009.01and2011.25;AACCMethod 32–45.01 and 32–50.01, and recently adopted by Codex Alimentarius as a Type I Method. However, in application of the method to a diverse range of food samples and particularly food ingredients, some limitations have been identified. One of the ongoing criticisms of this method was that the time of incubation with pancreatic a-amylase/amyloglucosidase mixture was 16 h, whereas the time for food to transit through the human small intestine was likely to be approximately 4 h. In the current work, we use an incubation time of 4 h, and have evaluated incubation conditions that yield resistant starch and dietary values in line with ileostomy results within this time frame. Problems associated with production, hydrolysis and chromatography of various oligosaccharides have been addressed resulting in a more rapid procedure that is directly applicable to all foods and food ingredients currently available. Keywords: Available carbohydrates / Codex Alimentarius / Dietary fibre determination / Enzymic / Non-digestible oligosaccharides / Resistant starch : Additional supporting information may be found in the online version of this article at the publisher’s web-site. -
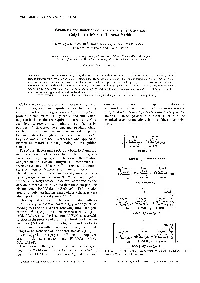
Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(Glcnach Tetrasaccharide
Polymer Journal, Vol. 30. No. 8, pp 653-658 ( 1998) Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(GlcNAch Tetrasaccharide Kazukiyo KoBAYASHI,t Shoko KAMIYA, Minoru MATSUYAMA,* Takeomi MURATA,* and Taichi Usur* Graduate School of' Engineering, Nagoya University, Chikusa, Nagoya 464-8603, Japan * Faculty of' Agriculture, Shizuoka University, Ohya, Shizuoka 422---8529, Japan (Received February 16, 1998) ABSTRACT: Tetrasaccharide Galfil--+4(GlcNAch was synthesized from N,N',N"-triacetylchitotriose (GlcNAc)s and lactose using transglycosylation with a P-o-galactosidase from Bacillus circu/ans. The reducing terminal of Galfil--+4(GlcNAclJ was oxidized and connected to p-vinylbenzylamine via amide linkage, and the resulting oligosaccharide-substituted styrene monomer was polymerized with the radical initiator, 2,2' -azobis(2-amidinopropane) dihydrochloride at 60"C. Glycopolystyrene was found to bind strongly with wheat germ agglutinin (WGA) and tomato (Lycopersicon esculentum) agglutinin (LEA) by inhibition of hemagglutination and double diffusion. KEY WORDS Oligosaccharides / Glycopolymers / Lectins / Enzymatic Synthesis/ Recognition / Cell surface carbohydrates from glycoproteins, glyco dimensional immunodiffusion in agar and inhibition of lipids, proteoglycans, and capsular polysaccharides play lectin-induced hemagglutination. Comparison was made important roles in biological events, 1.2 Carbohydrate using analogous homopolymers carrying N-acetyllactos protein interaction is usually weak and multivalent amine and chitooligosaccharides. Chart I illustrates the oligosaccharide chains are required to target cell surface chemical structures and abbreviations of these glycopoly carbohydrate receptors and inhibit host infection by mers. pathogens. 3 Glycopolymers carrying pendant oligo saccharide chains can be regarded as multivalent ligands known to interact strongly with lectins and antibodies, 4 · 5 Glycopolymers have been synthesized and applied as biomedical materials having biological recognition signals. -

Determination of Carbohydrates in Honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA
Determination of carbohydrates in honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA ABSTRACT RESULTS SAMPLE ANALYSIS METHOD ACCURACY Table 7. Adulteration parameters for HS6 adulterated with 10% SS1 through SS5. Purpose: To develop an HPAE-PAD method for the determination of carbohydrates in honey Honey sugar analysis Sample Recovery HS6 (Wild Mountain Honey) samples to evaluate their quality and to assess the possibility of adulteration. Separation Adulteration Honey sugars were separated using a Dionex CarboPac PA210-Fast-4μm column (150 × 4 mm) in Method accuracy was evaluated by measuring recoveries of 10 sugar standards spiked into honey Parameters 100% + 10% + 10% + 10% + 10% + 10% For this study, we purchased 12 commercial honey samples (Table 1) and analyzed them using Honey SS1 SS2 SS3 SS4 SS5 Methods: Separation of individual honey sugars was achieved on the recently introduced Thermo series with a Dionex CarboPac PA210 guard column (50 × 4 mm). The column selectivity allow samples. For spiking experiments, four honey samples were used (HS7–HS10) and spiked with a 10- HPAE-PAD. Figure 3 shows the representative chromatograms of 3 honey samples. For all 12 Glucose(G), mg/L 121 115 116 117 119 107 Scientific™ Dionex™ CarboPac™ PA210-Fast-4μm column. Carbohydrate detection was by pulsed carbohydrates to be separated with only a hydroxide eluent generated using an eluent generator. A sugar standard mix at two concentration levels. Figure 4 shows the representative chromatograms investigated honey samples, fructose and glucose (Peak 2 and Peak 3), were found to be the major Fructose(F), mg/L 127 115 115 116 126 116 amperometric detection (PAD) with a gold working electrode and, therefore, no sample derivatization solution of honey sugar standards was prepared and an aliquot (10 μL) of the solution was injected of unspiked and spiked honey sample HS7. -

Carbohydrates: Occurrence, Structures and Chemistry
Carbohydrates: Occurrence, Structures and Chemistry FRIEDER W. LICHTENTHALER, Clemens-Schopf-Institut€ fur€ Organische Chemie und Biochemie, Technische Universit€at Darmstadt, Darmstadt, Germany 1. Introduction..................... 1 6.3. Isomerization .................. 17 2. Monosaccharides ................. 2 6.4. Decomposition ................. 18 2.1. Structure and Configuration ...... 2 7. Reactions at the Carbonyl Group . 18 2.2. Ring Forms of Sugars: Cyclic 7.1. Glycosides .................... 18 Hemiacetals ................... 3 7.2. Thioacetals and Thioglycosides .... 19 2.3. Conformation of Pyranoses and 7.3. Glycosylamines, Hydrazones, and Furanoses..................... 4 Osazones ..................... 19 2.4. Structural Variations of 7.4. Chain Extension................ 20 Monosaccharides ............... 6 7.5. Chain Degradation. ........... 21 3. Oligosaccharides ................. 7 7.6. Reductions to Alditols ........... 21 3.1. Common Disaccharides .......... 7 7.7. Oxidation .................... 23 3.2. Cyclodextrins .................. 10 8. Reactions at the Hydroxyl Groups. 23 4. Polysaccharides ................. 11 8.1. Ethers ....................... 23 5. Nomenclature .................. 15 8.2. Esters of Inorganic Acids......... 24 6. General Reactions . ............ 16 8.3. Esters of Organic Acids .......... 25 6.1. Hydrolysis .................... 16 8.4. Acylated Glycosyl Halides ........ 25 6.2. Dehydration ................... 16 8.5. Acetals ....................... 26 1. Introduction replacement of one or more hydroxyl group (s) by a hydrogen atom, an amino group, a thiol Terrestrial biomass constitutes a multifaceted group, or similar heteroatomic groups. A simi- conglomeration of low and high molecular mass larly broad meaning applies to the word ‘sugar’, products, exemplified by sugars, hydroxy and which is often used as a synonym for amino acids, lipids, and biopolymers such as ‘monosaccharide’, but may also be applied to cellulose, hemicelluloses, chitin, starch, lignin simple compounds containing more than one and proteins. -

Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments
A Dissertation Titled: Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments from B. fragilis ATCC 25285/NCTC 9343 with Alternating Charges on Adjacent Monosaccharides by Pradheep Eradi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry ___________________________________________ Dr. Peter R. Andreana, PhD, Committee Chair ___________________________________________ Dr. Steve Sucheck, PhD, Committee Member ___________________________________________ Dr. Jianglong Zhu, PhD, Committee Member ___________________________________________ Dr. Amanda C. Bryant-Freidrich, PhD, Committee Member ___________________________________________ Dr. Cyndee Gruden, Dean College of Graduate Studies The University of Toledo May 2019 Copyright 2019 Pradheep Eradi This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Total Synthesis of Zwitterionic Bacterial Polysaccharide (PS A1) Antigen Fragments from B. fragilis ATCC 25285/NCTC 9343 with Alternating Charges on Adjacent Monosaccharides by Pradheep Eradi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry The University of Toledo May 2019 Zwitterionic polysaccharides (ZPSs) are a relatively new class of carbohydrate antigens, with a paradigm shifting property; they can activate CD4+ T-cells in the absence of lipids, peptide(s) or protein(s) upon MHC class II presentation. Up until now, various anaerobic bacteria are known to express ZPSs, for example, PS A1, PS A2 and PS B (Bacteroides fragilis), Sp1 (Streptococcus pneumoniae), CP5 and CP8 (Staphylococcus aureus) and O-chain antigen (Morganella morgani). Among all the afore mentioned ZPSs, Sp1 and PS A1 polysaccharides were the prime focus of research for the past few decades and their biological properties are very well-understood. -

Tetrasaccharide Glycoforms from Bacillus Anthracis Exosporium and Fragments Thereof
molecules Article Structure-Immunogenicity Relationship of α- and β-Tetrasaccharide Glycoforms from Bacillus anthracis Exosporium and Fragments Thereof Riccardo De Ricco 1, Christy L. Ventura 2, Filippo Carboni 1, Rina Saksena 3,†, Pavol Kováˇc 3 and Roberto Adamo 1,* ID 1 GSK, Research Centre, via Fiorentina 1, 53100 Siena, Italy; [email protected] (R.D.R.); fi[email protected] (F.C.) 2 Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; [email protected] 3 NIDDK, LBC, National Institutes of Health, Bethesda, MD 20892-0815, USA; [email protected] (R.S.); [email protected] (P.K.) * Correspondence: [email protected]; Tel.: +39-0577-539393 † Current address: Department of Chemistry, The University of New Mexico, Albuquerque, NM 87131, USA. Received: 12 July 2018; Accepted: 17 August 2018; Published: 20 August 2018 Abstract: The tetrasaccharide (2-O-methyl-4-(3-hydroxy-3-methylbutamido)-4,6-dideoxy-α-D- glucopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!3)-α-L-rhamnopyranosyl-(1!2)-L-rhamnopyranose) from the major exosporium protein (BclA) of Bacillus anthracis has been proposed as a target for development of diagnostics and immune therapy or prophylaxis. While the immunodominant character of the anthrose residue has been previously elucidated, the role of the stereochemical configuration of the downstream rhamnose is unknown. Because the linkage of this residue to the GlcNAc bridging the glycan and the protein is lost during isolation of the tetrasaccharide, its α- and β-glycoforms have been synthesized. Herein, we prepared neoglycoconjugates from a series of fragments of the tetrasaccharide, including the complete α- and β-tetrasaccharide glycoforms, a 2-demethoxylated version of the α-tetrasaccharide, and the α- and β-trirhamnosides and CRM197. -

Structures of Branched Dextrins Produced by Saccharifying A-Amylase of Bacillus Subtilis
/. Biochem., 72, 1219-1226 (1972) Structures of Branched Dextrins Produced by Saccharifying a-Amylase of Bacillus subtilis Kimio UMEKI and Takehiko YAMAMOTO Downloaded from https://academic.oup.com/jb/article/72/5/1219/878303 by guest on 29 September 2021 The Faculty of Science, Osaka City University, Osaka Received for publication, May 25, 1972 The branched dextrins produced from waxy rice starch ^-limit dextrin by Bacillus subtilis' saccharifying a-amylse [EC 3.2.1.1] were isolated by the multiple develop- ment paper chromatography and their chemical structures were investigated. The analyses using £-amylase [EC 3.2.1. 2], glucoamylase [EC 3.2.1.3], pullulanase, and pullulan a-1,4-glucoside hydrolase revealed that they were doubly branched dextrins with the following structures: e'-a-^'-a-glucosylmaltosylJ-maltotriose, 68-a-, 65-a- diglucosylmaltopentaose, and 68-a-{6*-a-glucosylmaltotriosyl)-maltotriose. The results were discussed in connection with the interior structure of /3-limit dextrin. Only a few papers have so far been published from each other (8, 9). on the action of a-amylase [EC 3.2.1.1] on The saccharifying a-amylase is character- branched substrates (1-3). French and his istic in that it hydrolyzes starch to produce coworkers have reported that the porcine reducing sugars of about twice as much as pancreatic a-amylase produces various branched those produced by the liquefying a-amylase dextrins as the limit dextrins (4). Our pre- and none of the hydrolysis products by the vious paper (5) has also shown that a-amylase saccharifying a-amylase are attacked by the of starch liquefying type from Bacillus subtilis liquefying a-amylase. -

Quantitative Changes in Various Sugar Concentrations During Fermentation
QUAriTri'AT:CVE GHAI'jGES 111 VARIOUS SUGAR GOIjCm'RATlOKS UJRIMG FEH'IiSm'ATlOII Oi<' DOUGliS, SPONGiiS AMD BRliT/ZS ^' Ting Jui Tan^ 3. S., Tai-.v-an Provincial ChTJungshing Universitj . A MSTEK'S THESIS subnitted in partial fulfilLTient of the req-uireiaent Tor the decree MSTitlR OF SCIENCE Food Science Depairtinsnt Ox Grain Science arid Ladiistiy KAIIS'IS STATK UNXVilRSI'i'Y Manhattan, Kansas 1963 ApTrov'i'id by: I'lajor Professor . ' - LD . /?(? . tSX table of coiitehts eitroduction 1 REVIEV7 OF LITER/iTUPJi; 2 I. The Carbohydrates of Ivlieat and VJheat Flour 2 II. Quantitative Determination of Sugars in Natural ............ Materials 5 III, The Fate of Sugars in Breadmaking ......... 11 ^lETHOD AND MATERIALS l6 I. Flour Samples l6 II. Formulation, Dough I-Iake-up and Balcing 16 III, Determination of Gas Production Rates 19 IV. Schedule of Sample Collection for Sugar Analyses ..... 20 V. Method of Ejctraction of Sugars from Dough, Brew, Sponge and Bread Crumb . , 21 VI. tiethods of Analyses , 21 RESULTS AND DISCUSSION 25 I. of Discussion the Experimental Methods , 25 II. Change in Total Reducing Sugars of the Extracts 31 III. Change of Concentration of Individual Sugars During Fermentation ........... ....... 32 IV. Rate of Gas Production 34 V. Residual Sugars in Bread 36 SU>2^SIRY AND CONCLUSION 37 ACKI^Oi-niEDGEMEIT Zfo LITERATURE CITED , ^ i^l ii LIST _0F TABLES I. The I-Iono- and Di- saccharide Content of Flour , 3 II, Changes of Sugar Content in Steeped and Germinated Ivheat , , , , 12 III, Size of Sar.ples Used in Detenrdnation of Gas Production Hates , . 19 IV. -

Structure and Metabolism of the Intracellular Polysaccharide of Corynebacterium. Don D
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 Structure and Metabolism of the Intracellular Polysaccharide of Corynebacterium. Don D. Mickey Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Mickey, Don D., "Structure and Metabolism of the Intracellular Polysaccharide of Corynebacterium." (1969). LSU Historical Dissertations and Theses. 1679. https://digitalcommons.lsu.edu/gradschool_disstheses/1679 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 70-9079 MICKEY, Don D., 1940- STRUCTURE AND METABOLISM OF THE INTRACELLULAR POLYSACCHARIDE OF CORYNEBACTERIUM. The Louisiana State University and Agricultural and Mechanical College, Ph.D., 1969 Microbiology University Microfilms, Inc., Ann Arbor, Michigan STRUCTURE AND METABOLISM OF THE INTRACELLULAR POLYSACCHARIDE OF CORYNEBACTERIUM A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Microbiology by Don D. Mickey B. S., Louisiana State University, 1963 August, 1969 ACKNOWLEDGMENT The author wishes to acknowledge Dr. M. D. Socolofsky for his guidance during the preparation of this dissertation. He also wishes to thank Dr. H. D. Braymer and Dr. A. D. Larson and other members of the Department of Microbiology for helpful advice given during various phases of this research. -

Nutritive Sweeteners from Corn Have Become America’S Premier Sweeteners
NutritiveNutritive SweetenersSweeteners FromFrom CornCorn CONTENTS Member Companies and Plant Locations ....................................... 2 Foreword .......................................................................................... 3 Historical Perspective ...................................................................... 4 Research and development orientation ....................................... 5 Technology aimed at needs .......................................................... 7 Growth, Development and Diversity ............................................. 7 CONTENTS Classification and Nutrition ............................................................ 9 Classification ................................................................................. 9 Corn sweeteners in nutrition ..................................................... 10 Technical Background ................................................................... 11 Corn starch ................................................................................. 11 Starch hydrolysis ........................................................................ 13 Crystalline dextrose .................................................................... 14 Dextrose isomerization .............................................................. 15 Manufacture ................................................................................... 17 Corn syrups ................................................................................ 17 Dried corn syrups ...................................................................... -

Oligosaccharides Disaccharides
1 Oligosaccharides Oligosaccharides are carbohydrates formed of 2-10 monosaccharide units covalently bonded to each other by glycosidic bonds. According to the number of sugar units, they are classified into disaccharides, trisaccharides and so on. Disaccharides Disaccharides are oligosaccharides formed of two monosaccharide units covalently bonded by glycosidic bond. Classification Disaccharides are classified according to the type of its monosaccharide unites into: 1- Homodisaccharides These are disaccharides in which the two monosaccharide units are the same e.g.: • Maltose which formed of two α-glucose units linked together by α- (1,4) glycosidic bond It is the major degradation product of starch It has a free aldehyde group so; it is a reducing sugar • Cellobiose which is formed of two units of β glucose linked together by β-(1,4) glycosidic bond. It has a free aldehyde group so; it is a reducing sugar 2- Heterodisaccharide These are disaccharides in which the two monosaccharide units are different e.g.: 2 • Sucrose which is formed of one α glucose molecule and one β fructose molecule linked by α-(1,2)β-glycosidic bond It is prevalent in cane sugar and beets. Sucrose is a non-reducing sugar as it contains no free aldehyde or ketone group. • Lactose is found exclusively in the milk It is formed of one β- galactose and one β- glucose linked together by β-(1,4) glycosidic bond. It has a free aldehyde group so; it is a reducing sugar. Trisaccharides Trisaccharides are oligosaccharides formed of three monosaccharide units covalently bonded to each other by glycosidic bonds.