Mobile Gait Analysis: from Prototype Towards Clinical Grade Wearable
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
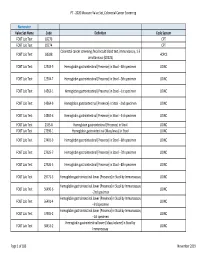
2020 Measure Value Set Colorectal Cancer Screening
PT ‐ 2020 Measure Value Set_Colorectal Cancer Screening Numerator Value Set Name Code Definition Code System FOBT Lab Test 82270 CPT FOBT Lab Test 82274 CPT Colorectal cancer screening; fecal occult blood test, immunoassay, 1‐3 FOBT Lab Test G0328 HCPCS simultaneous (G0328) FOBT Lab Test 12503‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐4th specimen LOINC FOBT Lab Test 12504‐7 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐5th specimen LOINC FOBT Lab Test 14563‐1 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐1st specimen LOINC FOBT Lab Test 14564‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐2nd specimen LOINC FOBT Lab Test 14565‐6 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐3rd specimen LOINC FOBT Lab Test 2335‐8 Hemoglobin.gastrointestinal [Presence] in Stool LOINC FOBT Lab Test 27396‐1 Hemoglobin.gastrointestinal [Mass/mass] in Stool LOINC FOBT Lab Test 27401‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐6th specimen LOINC FOBT Lab Test 27925‐7 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐7th specimen LOINC FOBT Lab Test 27926‐5 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐8th specimen LOINC FOBT Lab Test 29771‐3 Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay LOINC Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 56490‐6 LOINC ‐‐2nd specimen Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 56491‐4 LOINC ‐‐3rd specimen Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 57905‐2 -

9781441967237.Pdf
The Neurologic Diagnosis wwwwwwwwww Jack N. Alpert The Neurologic Diagnosis A Practical Bedside Approach Jack N. Alpert, MD St. Luke’s Episcopal Hospital Department of Neurology University of Texas Medical School at Houston Houston, TX, USA [email protected] ISBN 978-1-4419-6723-7 e-ISBN 978-1-4419-6724-4 DOI 10.1007/978-1-4419-6724-4 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2011941214 © Springer Science+Business Media, LLC 2012 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identifi ed as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein. Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) In Memory of Morris B. -

Physical Therapy Practice
Physical Therapy Practice THE MAGAZINE OF THE ORTHOPAEDIC SECTION, APTA VOL. 18, NO. 2 2006 ORTHOPAEDIC CARDON REHABILITATION PRODUCTS, INC.™ Wurlitzer Industrial Park, 908 Niagara Falls Blvd. North Tonawanda, NY 14120 Telephone: 1-800-944-7868 • Fax: 716-297-0411 E-mail: [email protected] THE ACCEPTED STANDARD OF PERFORMANCE The Cardon Mobilization Table . Going beyond the third dimension . Now available with the patented option which eliminates the use of flexion and rotation levers. This allows the therapist to perform advanced manual therapy techniques with complete confidence and comfort with an ergonomically friendly design. The unique design provides more efficient and smooth setup while providing superior patient comfort. The option enhances patient care by allowing unsurpassed opportunity for more preciseness of treatment and monitoring of segments and joints. he design and concepts make this “T the best mobilization table manufactured today.” Professor Freddy Kaltenborn Autho, Int’l Lecturer in Manual Therapy he various sections have minimum flex “T allowing very accurate application of specific manual therapy techniques.” Olaf Evjenth ES! I would like to preview the Author, Int’l Lecturer in Manual Therapy Y Cardon Mobilization Table. Please rush your 15 minute VHS video (for standard model): SEE FOR Name: Title: YOURSELF Clinic/Institution: THESE Address: OUTSTANDING City: State: Zip Code: Telephone: Signature: FEATURES: CARDON REHABILITATION PRODUCTS, INC.™ • Accurate localization of the vertebral segment Wurlitzer Industrial Park, 908 Niagara Falls Blvd. • Precision and versatility of technique North Tonawanda, NY 14120 • Absolute control of the mobilization forces Telephone: 1-800-944-7868 • Fax: 716-297-0411 • Excellent stability for manipulation. E-mail: [email protected] Orthopaedic Practice Vol. -

Neurological History and Physical Examination
emedicine.medscape.com eMedicine Specialties > Clinical Procedures > none Neurological History and Physical Examination Kalarickal J Oommen, MD, FAAN, Professor and Crofoot Chair of Epilepsy, Department of Neurology, Chief, Section of Epilepsy, Texas Tech University Health Sciences Center; Medical Director, Texas Tech University Health Sciences Center (TTUHSC) Covenant Comprehensive Epilepsy Center Updated: Nov 25, 2009 Neurological History "From the brain and the brain only arise our pleasures, joys, laughter and jests, as well as our sorrows, pains, griefs, and tears.... These things we suffer all come from the brain, when it is not healthy, but becomes abnormally hot, cold, moist or dry." —Hippocrates The Sacred Disease, Section XVII Taking the patient's history is traditionally the first step in virtually every clinical encounter. A thorough neurologic history allows the clinician to define the patient's problem and, along with the result of physical examination, assists in formulating an etiologic and/or pathologic diagnosis in most cases.[1 ] Solid knowledge of the basic principles of the various disease processes is essential for obtaining a good history. As Goethe stated, "The eyes see what the mind knows." To this end, the reader is referred to the literature about the natural history of diseases. The purpose of this article is to highlight the process of the examination rather than to provide details about the clinical and pathologic features of specific diseases. The history of the presenting illness or chief complaint should -

Movement Disorders and Gait Disturbances
Movement disorders and gait disturbances Kovács Norbert PTE ÁOK Neurológiai Klinika Pécs 1 MD pathophysiology z Genetic mutation or environmental injury of basal ganglia functioning z Pallidum, thalamus, subthalamic nucleus, caudate nucleus, pedunculopintine nucleus 2 Vitek JL. Mov Disord 2002;17(Supp 3):S49-62 Phenomenology in MD Hyperkinetic Isokinetic Hypokinetic • Tremor (regular) • Ataxia • Rigidity • Chorea • Bradykinesia • Ballism • Hypokinesia • Dystonia • Athetosis • Myoclonus (jerky) • Tic (jerky) 3 Hyperkinetic movements 4 Tremor classification More or less regular, sinusoid movements Any body parts can be affected (e.g. limbs, neck, trunc, vocal cords) Classification: • Intensity (invisible, barely visible, moderate, severe) • Frequency (slow or fast) • Position – Rest tremor (e.g. Parkinsonism) – Postural tremor (e.g. hyperthyroidism) – Kinetic tremor (e.g. essential tremor) – Intention tremor (e.g. cerebellar tremor) 5 Rest tremor Cognition (e.g. counting), gait or talking about the disease 6 usually increases the amplitude Intention tremor The tremor amplitude is the highest at the target. Usually 7 caused by cerebellar problems. Postural –kinetic tremor 8 Postural –kinetic tremor 9 Essential tremor is the most frequent cause of kinetic tremor. Postural –kinetic tremor 10 Always examine water drinking, writing and tableware use -- QoL Deep brain stimulation for tremor 11 Chorea The word chorea denotes rapid irregular muscle jerks that occur involuntarily and unpredictably in different parts of the body. Most important cause is12 Parkinson’s disease Ballism Large involuntary movements involving the whole extremity. Usually accompanies the chorea. Vascular lesion e.g. in the area of subthalamic13 nucleus can produce Athetosis abnormal movements that are slow, sinuous, and writhing in character. 14 Dystonia • Not a disease, it is a syndrome • Involuntary phasic, movement and/or • Sustained, involuntary, abnormal muscle contractions. -

Evaluating the Child with Unsteady Gait
Review Article Evaluating the child with unsteady gait Mohammed M. Jan, MBChB, FRCP(C). ABSTRACT From the Department of Pediatrics, King Abdul-Aziz University Hospital, Jeddah, Kingdom of Saudi Arabia. Address correspondence and reprint request to: Prof. Mohammed M. S. Jan, Department of Pediatrics, King Abdul-Aziz University يعتبر خلل التوازن أثناء املشي من اﻷعراض الشائعة لدى اﻷطفال Hospital, PO Box 80215, Jeddah 21589, Kingdom of Saudi (Arabia. Tel. +996 (2) 6401000 Ext. 20208. Fax. +996 (2 بقسم الطوارئ واﻷعصاب. تتعدد أسباب خلل التوازن، ولكن E-mail: [email protected] .6403975 من أهم اولويات التقييم اﻷولى هو التأكد من عدم وجود التهاب أو ورم بالدماغ. التعرف علي املسببات احلميدة والغير عصبية ًأيضا eurological disorders are common in Saudi Arabia مهم لتفادى القيام بفحوصات متعددة دون احلاجة إليها أو تنومي Naccounting for up to 30% of all consultations to املريض باملستشفى. في هذه املقالة النقدية نقدم مراجعة حديثة pediatrics.1 Trauma, ingestion, and acute neurological عن تقييم الطفل املصاب بخلل التوازن مع مناقشة الفحوصات disorders are common, mainly as a result of improper الﻻزمة والعﻻج. قد يكون خلل التوازن ناجت عن مرض باملخيخ safety practices of many parents.2 Consanguineous أو مشكلة حسية، ًعلما بأن أمراض املخيخ قد تكون حادة، marriages also add to the problem, resulting in مزمنة، متدهورة، أو متقطعة. وتتعدد أسباب هذه املشكلة و increased prevalence of many inherited and genetic منها اﻹصابات، اﻻلتهابات، أمراض اﻻستقﻻب، العيوب اخللقية، neurological disorders.3,4 Unsteadiness and ataxia are واﻷورام. أما أسباب خلل التوازن الناجت عن مشاكل اﻹحساس relatively common neurological presentations of a فيكون بسبب تأثر في اﻷعصاب الطرفية أو احلبل الشوكي. -

Gait Disorders
What are the classical Gait Patterns for the Following Conditions? • Alzheimers Disease Gait Disorders • Hemiparetic Stroke • Parkinsons Disease T.Masud • Osteomalacia Nottingham University Hospitals NHS Trust • Lateral popliteal nerve palsy University of Nottingham University of Derby • Knee OA University of Southern Denmark • Vitamin B12 deficiency with dorsal column loss Statistical summaries of risk factors for falls From cohort studies- Perell 2001 RISK FACTOR Mean RR/OR Range Muscle weakness 4.4 (1.5-10.3) Falls history 3.0 (1.7-7.0) Gait deficit 2.9 (1.3-5.6) Balance deficit 2.9 (1.6-5.4) Use of assistive devices 2.6 (1.2-4.6) Visual deficit 2.5 (1.6-3.5) Arthritis 2.4 (1.9-2.7) Impaired ADLs 2.3 (1.5-3.1) Depression 2.2 (1.7-2.5) Cognitive impairment 1.8 (1.0-2.3) Age > 80 1.7 (1.1-2.5) Simple Model for Balance Balance Vision FALLS Vestibular Musculo- skeletal Proprioception Tactile sensation Activity & environmental hazards CNS Gait cycle [weight bearing] [progress] Running: stance 50% - swing 50%, then Asymmetry no double support period Stance phase Condition Disabled: increased bilateral stance phase Pain, weakness to increase double support period Impaired balance: vestibular, cerebellum dysfunction Clinical gait analysis Pattern Recognition of Gait Pattern recognition Hemiplegic Parkinsonian - Most quickly, recall from memory Apraxic Structured Approach Neuropathic - Hypothetico-deductive Ataxic - Basic gait knowledge / Anatomy Waddling Exhaustive strategy Spastic - Comprehensive and systematic evaluation Hyperkinetic Antalgic Gait Disorder in Older People High Level Gait Disorders by level of Sensorimotor Deficit Frontal Related • Apraxic •Cerebrovascular • Magnetic Low • Freezing High Middle •Dementia Level Level Level From- Alexander, Goldberg, Cleveland Clinic J Med 2005; 72: 592-600 High Level Gait Disorders High Level Gait Disorders Frontal Related Frontal Related •Cerebrovascular •Cerebrovascular •Dementia •Dementia •N.P. -

University of Florida Thesis Or Dissertation Formatting
EXAMINING THE NEXUS AMONGST JOINT DEGENERATION, DISABILITY, AND CHRONIC PAIN USING A RODENT MODEL OF POST-TRAUMATIC KNEE OSTEOARTHRITIS By HEIDI ELISE KLOEFKORN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2016 © 2016 Heidi Elise Kloefkorn To my husband ACKNOWLEDGMENTS While a doctoral degree is only conferred to one person, the degree is earned due to the efforts of many. I would like to first thank my husband, Spencer Adams, and my parents, Randy and Leigh Ann Kloefkorn. Their support, love, and patience are invaluable. I would like to thank my chair, Kyle Allen, for seeing my potential, encouraging me to succeed, and teaching me that failure is always an option. With his guidance (and a healthy dose of snark), I have grown as a scientist and academic with a solid foundation to continue my career. I am most grateful that, when it came to his efforts to improve my writing skills, failure was NOT an option. I would also like to thank my lab mates for their comradery and expertise. They have unfailingly provided help, perspective, advice, and laughter at just the right moment. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS .................................................................................................. 4 LIST OF TABLES ............................................................................................................ 8 LIST OF FIGURES ......................................................................................................... -

Pediatric Orthopedics in Practice, DOI 10.1007/978-3-662-46810-4, © Springer-Verlag Berlin Heidelberg 2015 880 Backmatter
879 Backmatter Subject Index – 880 F. Hefti, Pediatric Orthopedics in Practice, DOI 10.1007/978-3-662-46810-4, © Springer-Verlag Berlin Heidelberg 2015 880 Backmatter Subject index Bold letters: Principal article Italics: Illustrations A Acetylsalicylic acid 303, 335 Adolescent scoliosis Amyloidosis 663 Acheiropodia 804 7 Scoliosis Amyoplasia 813–814 Abducent nerve paresis 752, Achievement by proxy 10, 11 AFO 7 Ankle Foot Orthosis Anaerobes 649, 652, 657 816 Achilles tendon Aggrecan 336, 367, 762 ANA 7 antinuclear antibodies Abducted pes planovalgus – lengthening 371, 426, 431, aggressive osteomyelitis Analysis, gait 488, 490–497 433, 434, 436, 439, 443, 464, 7 osteomyelitis, aggressive 7 Gait analysis Abduction contracture 468, 475, 485, 487–490, 493, Agonist 281, 487, 492, 493, Anchor 169, 312, 550, 734 7 contracture 496, 816, 838, 840 495, 498, 664, 832, 835, 840, Andersen classification abduction pants 219–221 – shortening 358, 418, 431, 868 7 classification, Andersen Abduction splint 212, 218–221, 433, 464, 465, 467, 468, 475, Ahn classification 366 Andry, Nicolas 21, 22 248, 850 489, 496, 838 Aitken classification (congenital Anesthesia 26, 38, 135, 154, Abduction Achondrogenesis 750, 751, femoral deficiency ) 7 classi- 162, 174, 221, 243, 247, 248, – hip 195, 198, 199, 212, 213, 756, 758–760, 769 fication, femoral deficiency 255, 281, 303, 385, 386, 400, 214, 218, 219, 220, 221, Achondroplasia 56, 163, 166, Akin osteotomy 477, 479 500, 506, 559, 568, 582–585, 241–245, 247, 248, 251, 255, 242, 270, 271, 353, 409, 628, Albers-Schönberg -

The Limping Child
The Limping Child Todd Milbrandt, MD Division Chair Pediatric Orthopaedics Mayo Clinic‐Rochester Faculty Disclosure • No disclosures relevant to this talk Practice Gap • Primary Care Providers are faced with limping children everyday • The differential is long and can be confusing. • By creating a guiding algorithm, this can be a manageable problem. Objectives • Develop a differential diagnosis for the limping child • Briefly describe the diagnostic and therapeutic options for those conditions Limps defined • A deviation in a child’s walking pattern • More commonly unilateral • Reasons fall into one of three categories • Pain • Weakness • Structural abnormality Painful conditions • Osteoarticular infection • Neoplasia • Trauma • Fracture Weak Conditions •Neuromuscular • Cerebral palsy •Muscle weakness • Muscular Dystrophy Structural Condition • Leg length inequality • Joint stiffness • Articular surface deformity Infection‐Septic Arthritis •Who? • Young children and infants •How present? • Joint pain, involuntary guarding, and muscle spasm • Pseudo paralysis • May be toxic and febrile • But may not be!! Infection‐Septic Arthritis • How Diagnose? • Only definitive answer is ASPIRATION • Hip‐U/S can guide if leg pain due to septic arthritis • Must have a high index of suspicion • Labs help • If WBC>12,000, ESR>40, inability to bear wt, and fever>38.5 C, then septic arthritis >90% • Treatment • Surgical Drainage • Remember the Psoas abscess that can mimic the septic arthritis!!!! Infection‐Osteomyelitis •Who? • From neonate to 10 years old -

Gait Disorders in Older Adults Neil B
I'I{()(;IUSS IN (;1· RIA I RICS Gait Disorders in Older Adults Neil B. Alexander, MD oth aging and diseases that are more prevalent with aging and mortality attributable to congestive heart failure. IS Com Baffect how older adults walk. This review addresses the fortable walking speed is a better predictor of cardiac index evaluation and treatment of gait disorders in older adults. and functional status in patients with chronic heart failure The focus is on comfortable gait, the most common clinically and lung disease than treadmill or cycle ergometry exercise evaluated aspect of walking. testing.18,19 In patients with chronic lung disease, 6- and 12-minute walking tests are useful measures of response to GAIT AND DISABILITY exercise training and medication adminstration in most,19-23 From 8 to 19% of noninstitutionalized older adults have but not all,24,2$ studies. The relationship between walking difficulty walking or require the assistance of another person tests and pulmonary function tests are, however, inconsis or special equipment to walk. J,2 Walking disability increases tent,26-28 and there may also be a practice effect in walk 27 29 with age. For example, 6% of female East Boston respon time. - dents aged 65 to 69, compared with 38% of those aged 85 Other gait measures also correlate with functional out and older, need assistance in walking.:' Limitations in walk come. Walking cadence, as measured by the number of steps ing can be seen in as many as 63% of nursing home resi walked per minute, correlates with SIP scores.30Stride length dents." In older adults with diseases such as arthritis, 35% and the symmetry of swing duration in the unaffected versus admit to difficulty in walking one-quarter mile:' the affected leg correlate well with extent of motor recovery 31,32 Measures such as gait speed provide an index of func in hemiplegic patients. -

Differential Diagnosis in Neurology and Neurosurgery
I Differential Diagnosis in Neurology and Neurosurgery A Clinician’s Pocket Guide Sotirios A. Tsementzis, M.D., Ph.D. Professor and Chairman of Neurosurgery Director of the Neurosurgical Institute University of Ioannina Medical School Ioannina, Greece 16 Illustrations Thieme Stuttgart · New York 2000 Tsementzis, Differential Diagnosis in Neurology and Neurosurgery © 2000 Thieme All rights reserved. Usage subject to terms and conditions of license. II Library of Congress Cataloging-in-Publication Data Tsementzis, S. A. Differential diagnosis in neurosurgery / Sotirios A. Tsementzis. p. cm. Includes bibliographical references and index. ISBN 3-13-116151-5. – ISBN 0-86577-830-2 1. Nervous system–Surgery–Diagnosis Handbooks, manuals, etc. 2. Diagnosis, Differential Handbooks, manuals, etc. I. Title. [DNLM: 1. Nervous System Diseases–diagnosis. 2. Diagnosis, Differential. 3. Neurologic Examination. 4. Signs and Symptoms. WL 141 T881d 1999] RC348.T84 1999 616.8’0475–dc21 DNLM/DLC for Library of Congress 99-23138 CIP Cover drawing by Cyclus, Stuttgart Any reference to or mention of manufac- turers or specific brand names should not be Important Note: Medicine is an ever- interpreted as an endorsement or advertise- changing science undergoing continual ment for any company or product. development. Research and clinical ex- Some of the product names, patents, and reg- perience are continually expanding our istered designs referred to in this book are in knowledge, in particular our knowledge fact registered trademarks or proprietary of proper treatment and drug therapy. names even though specific reference to this Insofar as this book mentions any dosage fact is not always made in the text. Therefore, or application, readers may rest assured the appearance of a name without designa- that the authors, editors, and publishers tion as proprietary is not to be construed as a have made every effort to ensure that representation by the publisher that it is in such references are in accordance with the public domain.