Functional Gait Disorders a Sign-Based Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multiplex Families with Multiple System Atrophy
ORIGINAL CONTRIBUTION Multiplex Families With Multiple System Atrophy Kenju Hara, MD, PhD; Yoshio Momose, MD, PhD; Susumu Tokiguchi, MD, PhD; Mitsuteru Shimohata, MD, PhD; Kenshi Terajima, MD, PhD; Osamu Onodera, MD, PhD; Akiyoshi Kakita, MD, PhD; Mitsunori Yamada, MD, PhD; Hitoshi Takahashi, MD, PhD; Motoyuki Hirasawa, MD, PhD; Yoshikuni Mizuno, MD, PhD; Katsuhisa Ogata, MD, PhD; Jun Goto, MD, PhD; Ichiro Kanazawa, MD, PhD; Masatoyo Nishizawa, MD, PhD; Shoji Tsuji, MD, PhD Background: Multiple system atrophy (MSA) has Results: Consanguineous marriage was observed in 1 been considered a sporadic disease, without patterns of of 4 families. Among 8 patients, 1 had definite MSA, 5 inheritance. had probable MSA, and 2 had possible MSA. The most frequent phenotype was MSA with predominant parkin- Objective: To describe the clinical features of 4 multi- sonism, observed in 5 patients. Six patients showed pon- plex families with MSA, including clinical genetic tine atrophy with cross sign or slitlike signal change at aspects. the posterolateral putaminal margin or both on brain mag- netic resonance imaging. Possibilities of hereditary atax- Design: Clinical and genetic study. ias, including SCA1 (ataxin 1, ATXN1), SCA2 (ATXN2), Machado-Joseph disease/SCA3 (ATXN1), SCA6 (ATXN1), Setting: Four departments of neurology in Japan. SCA7 (ATXN7), SCA12 (protein phosphatase 2, regula- tory subunit B,  isoform; PP2R2B), SCA17 (TATA box Patients: Eight patients in 4 families with parkinson- binding protein, TBP) and DRPLA (atrophin 1; ATN1), ism, cerebellar ataxia, and autonomic failure with age at ␣ onset ranging from 58 to 72 years. Two siblings in each were excluded, and no mutations in the -synuclein gene family were affected with these conditions. -

Friedreich Ataxia Mouse Models with Progressive Cerebellar and Sensory Ataxia Reveal Autophagic Neurodegeneration in Dorsal Root Ganglia
The Journal of Neuroscience, February 25, 2004 • 24(8):1987–1995 • 1987 Neurobiology of Disease Friedreich Ataxia Mouse Models with Progressive Cerebellar and Sensory Ataxia Reveal Autophagic Neurodegeneration in Dorsal Root Ganglia Delphine Simon,1 Herve´ Seznec,1 Anne Gansmuller,1 Nade`ge Carelle,1 Philipp Weber,1 Daniel Metzger,1 Pierre Rustin,2 Michel Koenig,1 and He´le`ne Puccio1 1Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire, Centre National de la Recherche Scientifique/Institut National de la Sante´ et de la Recherche Me´dicale (INSERM)/Universite´ Louis Pasteur, 67404 Illkirch cedex, CU de Strasbourg, France, and 2Unite´ de Recherches sur les Handicaps Ge´ne´tiques de l’Enfant, INSERM U393, Hoˆpital Necker-Enfants Malades, 75015 Paris, France Friedreich ataxia (FRDA), the most common recessive ataxia, is characterized by degeneration of the large sensory neurons of the spinal cord and cardiomyopathy. It is caused by severely reduced levels of frataxin, a mitochondrial protein involved in iron–sulfur cluster (ISC) biosynthesis. Through a spatiotemporally controlled conditional gene-targeting approach, we have generated two mouse models for FRDA that specifically develop progressive mixed cerebellar and sensory ataxia, the most prominent neurological features of FRDA. Histological studies showed both spinal cord and dorsal root ganglia (DRG) anomalies with absence of motor neuropathy, a hallmark of the human disease. In addition, one line revealed a cerebellar granule cell loss, whereas both lines had Purkinje cell arborization defects. These lines represent the first FRDA models with a slowly progressive neurological degeneration. We identified an autophagic process as the causative pathological mechanism in the DRG, leading to removal of mitochondrial debris and apparition of lipofuscin deposits. -

Scienti®C Review Spastic Movement Disorder
Spinal Cord (2000) 38, 389 ± 393 ã 2000 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/00 $15.00 www.nature.com/sc Scienti®c Review Spastic movement disorder V Dietz*,1 1Paracare, Paraplegic Centre of the University Hospital Balgrist, ZuÈrich, Switzerland This review deals with the neuronal mechanisms underlying spastic movement disorder, assessed by electrophysiological means with the aim of ®rst, a better understanding of the underlying pathophysiology and second, the selection of an adequate treatment. For the patient usually one of the ®rst symptoms of a lesion within the central motor system represents the movement disorder, which is most characteristic during locomotion in patients with spasticity. The clinical examination reveals exaggerated tendon tap re¯exes and increased muscle tone typical of the spastic movement disorder. However, today we know that there exists only a weak relationship between the physical signs obtained during the clinical examination in a passive motor condition and the impaired neuronal mechanisms being in operation during an active movement. By the recording and analysis of electrophysiological and biomechanical parameters during a functional movement such as locomotion, the signi®cance of, for example, impaired re¯ex behaviour or pathophysiology of muscle tone and its contribution to the movement disorder can reliably be assessed. Consequently, an adequate treatment should not be restricted to the cosmetic therapy and correction of an isolated clinical parameter but should be based on the pathophysiology and signi®cance of the mechanisms underlying the disorder of functional movement which impairs the patient. Spinal Cord (2000) 38, 389 ± 393 Keywords: spinal cord injury; spasticity; electrophysiological recordings; treatment Introduction Movement disorders are prominent features of impaired strength of electromyographic (EMG) activation of function of the motor systems and are frequently best antagonistic leg muscles as well as intrinsic and passive re¯ected during gait. -
Pediatrics-EOR-Outline.Pdf
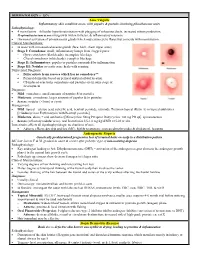
DERMATOLOGY – 15% Acne Vulgaris Inflammatory skin condition assoc. with papules & pustules involving pilosebaceous units Pathophysiology: • 4 main factors – follicular hyperkeratinization with plugging of sebaceous ducts, increased sebum production, Propionibacterium acnes overgrowth within follicles, & inflammatory response • Hormonal activation of pilosebaceous glands which may cause cyclic flares that coincide with menstruation Clinical Manifestations: • In areas with increased sebaceous glands (face, back, chest, upper arms) • Stage I: Comedones: small, inflammatory bumps from clogged pores - Open comedones (blackheads): incomplete blockage - Closed comedones (whiteheads): complete blockage • Stage II: Inflammatory: papules or pustules surrounded by inflammation • Stage III: Nodular or cystic acne: heals with scarring Differential Diagnosis: • Differentiate from rosacea which has no comedones** • Perioral dermatitis based on perioral and periorbital location • CS-induced acne lacks comedones and pustules are in same stage of development Diagnosis: • Mild: comedones, small amounts of papules &/or pustules • Moderate: comedones, larger amounts of papules &/or pustules • Severe: nodular (>5mm) or cystic Management: • Mild: topical – azelaic acid, salicylic acid, benzoyl peroxide, retinoids, Tretinoin topical (Retin A) or topical antibiotics [Clindamycin or Erythromycin with Benzoyl peroxide] • Moderate: above + oral antibiotics [Minocycline 50mg PO qd or Doxycycline 100 mg PO qd], spironolactone • Severe (refractory nodular acne): oral -

Analyzing Pterosaur Ontogeny and Sexual Dimorphism with Multivariate Allometry Erick Charles Anderson [email protected]
Marshall University Marshall Digital Scholar Theses, Dissertations and Capstones 2016 Analyzing Pterosaur Ontogeny and Sexual Dimorphism with Multivariate Allometry Erick Charles Anderson [email protected] Follow this and additional works at: http://mds.marshall.edu/etd Part of the Animal Sciences Commons, Ecology and Evolutionary Biology Commons, and the Paleontology Commons Recommended Citation Anderson, Erick Charles, "Analyzing Pterosaur Ontogeny and Sexual Dimorphism with Multivariate Allometry" (2016). Theses, Dissertations and Capstones. 1031. http://mds.marshall.edu/etd/1031 This Thesis is brought to you for free and open access by Marshall Digital Scholar. It has been accepted for inclusion in Theses, Dissertations and Capstones by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected], [email protected]. ANALYZING PTEROSAUR ONTOGENY AND SEXUAL DIMORPHISM WITH MULTIVARIATE ALLOMETRY A thesis submitted to the Graduate College of Marshall University In partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences by Erick Charles Anderson Approved by Dr. Frank R. O’Keefe, Committee Chairperson Dr. Suzanne Strait Dr. Andy Grass Marshall University May 2016 i ii ii Erick Charles Anderson ALL RIGHTS RESERVED iii Acknowledgments I would like to thank Dr. F. Robin O’Keefe for his guidance and advice during my three years at Marshall University. His past research and experience with reptile evolution made this research possible. I would also like to thank Dr. Andy Grass for his advice during the course of the research. I would like to thank my fellow graduate students Donald Morgan and Tiffany Aeling for their support, encouragement, and advice in the lab and bar during our two years working together. -

A Review on Parkinson's Disease Treatment
Lee et al. Neuroimmunol Neuroinflammation 2021;8:[Online First] Neuroimmunology DOI: 10.20517/2347-8659.2020.58 and Neuroinflammation Review Open Access A review on Parkinson’s disease treatment Tori K. Lee, Eva L. Yankee Department of Biology, Pacific Union College, Angwin, CA 94508, USA. Correspondence to: Tori K. Lee, Department of Biology, Pacific Union College, 1 Angwin Ave, Angwin, CA 94508, USA. E-mail: [email protected] How to cite this article: Lee TK, Yankee EL. A review on Parkinson’s disease treatment. Neuroimmunol Neuroinflammation 2021;8:[Online First]. http://dx.doi.org/10.20517/2347-8659.2020.58 Received: 1 Oct 2020 First Decision: 1 Dec 2020 Revised: 15 Dec 2020 Accepted: 24 Dec 2020 Available online: 25 Jan 2021 Academic Editors: Athanassios P. Kyritsis, Backil Sung Copy Editor: Monica Wang Production Editor: Jing Yu Abstract Parkinson’s disease (PD) is a neurodegenerative illness and has a common onset between the ages of 55 and 65 years. There is progressive development of both motor and non-motor symptoms, greatly affecting one’s overall quality of life. While there is no cure, various treatments have been developed to help manage the symptoms of PD. Management of PD is a growing field and targets new treatment methods, as well as improvements to old ones. Pharmacological, surgical, and therapeutic treatments have allowed physicians to treat not only the main motor symptoms of PD, but target patient-specific problems as they arise. This review discusses both the established and new possibilities for PD treatment that can provide patient-specific care and mitigate side effects for common treatments. -

Myelopathy—Paresis and Paralysis in Cats
Myelopathy—Paresis and Paralysis in Cats (Disorder of the Spinal Cord Leading to Weakness and Paralysis in Cats) Basics OVERVIEW • “Myelopathy”—any disorder or disease affecting the spinal cord; a myelopathy can cause weakness or partial paralysis (known as “paresis”) or complete loss of voluntary movements (known as “paralysis”) • Paresis or paralysis may affect all four limbs (known as “tetraparesis” or “tetraplegia,” respectively), may affect only the rear legs (known as “paraparesis” or “paraplegia,” respectively), the front and rear leg on the same side (known as “hemiparesis” or “hemiplegia,” respectively) or only one limb (known as “monoparesis” or “monoplegia,” respectively) • Paresis and paralysis also can be caused by disorders of the nerves and/or muscles to the legs (known as “peripheral neuromuscular disorders”) • The spine is composed of multiple bones with disks (intervertebral disks) located in between adjacent bones (vertebrae); the disks act as shock absorbers and allow movement of the spine; the vertebrae are named according to their location—cervical vertebrae are located in the neck and are numbered as cervical vertebrae one through seven or C1–C7; thoracic vertebrae are located from the area of the shoulders to the end of the ribs and are numbered as thoracic vertebrae one through thirteen or T1–T13; lumbar vertebrae start at the end of the ribs and continue to the pelvis and are numbered as lumbar vertebrae one through seven or L1–L7; the remaining vertebrae are the sacral and coccygeal (tail) vertebrae • The brain -

Efficacy of Rhythmic Auditory Stimulation on Ataxia and Functional Dependence Post- Cerebellar Stroke" (2020)
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale School of Medicine Physician Associate Program Theses School of Medicine 5-22-2020 Efficacy of Rhythmicudit A ory Stimulation on Ataxia and Functional Dependence Post-Cerebellar Stroke Kaitlin Fitzgerald Yale Physician Associate Program, [email protected] Follow this and additional works at: https://elischolar.library.yale.edu/ysmpa_theses Recommended Citation Fitzgerald, Kaitlin, "Efficacy of Rhythmic Auditory Stimulation on Ataxia and Functional Dependence Post- Cerebellar Stroke" (2020). Yale School of Medicine Physician Associate Program Theses. 13. https://elischolar.library.yale.edu/ysmpa_theses/13 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale School of Medicine Physician Associate Program Theses by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. EFFICACY OF RHYTHMIC AUDITORY STIMULATION ON ATAXIA AND FUNCTIONAL DEPENDENCE POST-CEREBELLAR STROKE A Thesis Presented to The Faculty of the School of Medicine Yale University In Candidacy for the degree of Master of Medical Science May 2020 Kaitlin Fitzgerald, PA-SII Dr. Diana Richardson, MD Class of 2020 Assistant Clinical Professor Yale Physician Associate Program. Yale School of Medicine, Neurology i Table of Contents ABSTRACT -

Running Birds from Quail to Ostrich Prioritise Leg Safety and Economy
© 2014. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2014) 217, 3786-3796 doi:10.1242/jeb.102640 RESEARCH ARTICLE Don’t break a leg: running birds from quail to ostrich prioritise leg safety and economy on uneven terrain Aleksandra V. Birn-Jeffery1,*,‡, Christian M. Hubicki2,‡, Yvonne Blum1, Daniel Renjewski2, Jonathan W. Hurst2 and Monica A. Daley1,§ ABSTRACT Daley, 2012; Dial, 2003; Jindrich et al., 2007; Rubenson et al., Cursorial ground birds are paragons of bipedal running that span a 2004). These athletes span the broadest body mass range among 500-fold mass range from quail to ostrich. Here we investigate the extant bipeds, over 500-fold from quail to ostrich. Birds thus provide task-level control priorities of cursorial birds by analysing how they a natural animal model for understanding the functional demands of negotiate single-step obstacles that create a conflict between body striding bipedalism and how these demands change with body size stability (attenuating deviations in body motion) and consistent leg (Gatesy and Biewener, 1991; Hutchinson and Garcia, 2002; Roberts force–length dynamics (for economy and leg safety). We also test the et al., 1998a). hypothesis that control priorities shift between body stability and leg Here, we ask two questions fundamental to locomotor behaviour: safety with increasing body size, reflecting use of active control to (1) what are the task-level leg control priorities of running animals; overcome size-related challenges. Weight-support demands lead to and (2) how do these priorities vary with terrain and body size? a shift towards straighter legs and stiffer steady gait with increasing Running animals must control their legs to balance numerous, body size, but it remains unknown whether non-steady locomotor sometimes conflicting, task-level demands including minimising priorities diverge with size. -

Neurologic Outcomes in Friedreich Ataxia: Study of a Single-Site Cohort E415
Volume 6, Number 3, June 2020 Neurology.org/NG A peer-reviewed clinical and translational neurology open access journal ARTICLE Neurologic outcomes in Friedreich ataxia: Study of a single-site cohort e415 ARTICLE Prevalence of RFC1-mediated spinocerebellar ataxia in a North American ataxia cohort e440 ARTICLE Mutations in the m-AAA proteases AFG3L2 and SPG7 are causing isolated dominant optic atrophy e428 ARTICLE Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy revisited: Genotype-phenotype correlations of all published cases e434 Academy Officers Neurology® is a registered trademark of the American Academy of Neurology (registration valid in the United States). James C. Stevens, MD, FAAN, President Neurology® Genetics (eISSN 2376-7839) is an open access journal published Orly Avitzur, MD, MBA, FAAN, President Elect online for the American Academy of Neurology, 201 Chicago Avenue, Ann H. Tilton, MD, FAAN, Vice President Minneapolis, MN 55415, by Wolters Kluwer Health, Inc. at 14700 Citicorp Drive, Bldg. 3, Hagerstown, MD 21742. Business offices are located at Two Carlayne E. Jackson, MD, FAAN, Secretary Commerce Square, 2001 Market Street, Philadelphia, PA 19103. Production offices are located at 351 West Camden Street, Baltimore, MD 21201-2436. Janis M. Miyasaki, MD, MEd, FRCPC, FAAN, Treasurer © 2020 American Academy of Neurology. Ralph L. Sacco, MD, MS, FAAN, Past President Neurology® Genetics is an official journal of the American Academy of Neurology. Journal website: Neurology.org/ng, AAN website: AAN.com CEO, American Academy of Neurology Copyright and Permission Information: Please go to the journal website (www.neurology.org/ng) and click the Permissions tab for the relevant Mary E. -

Clinical Decision Making in Neurology
CLINICAL DECISION MAKING IN NEUROLOGY The Neurological Examination The goals of the neurological examination are to detect the presence of a neurologic disease and to determine the location of the lesion within the nervous system. The neurologic examination should be performed in a systematic manner in order to assure that the animal's neurologic status is completely evaluated. The parts of a customary neurologic examination include evaluation of the head (mentation and cranial nerves), gait, limbs (postural reactions and spinal reflexes) and pain/nociception (normal and abnormal pain responses). Gait Evaluation I find gait evaluation the most useful and important part of the neurologic exam. Clinical evaluation of gait usually involves observation of the animal's movements while walking. This is best accomplished by having a handler walk the animal over a flat, non-slippery area. I typically evaluate gait by having a handler walk the animal approximately 100 feet, away and back, as well as circling clockwise and counterclockwise. I have the animal short on the leash and under good control and have the dog typically walk slowly. I may have them walk on and off the curb. Overall, evaluation is made by watching for paresis, ataxia, stride length, lameness, stiffness, spasticity, dysmetria and shuffling or scuffing of limbs or digits. I also note position of head and tail during gait evaluation. Gait analysis is broken down between the axial and appendicular skeleton. The axial vertebral column is made up of many joints and is divided into anatomical segments. The axial portion includes the head, neck, thoracic, lumbar, lumbar/pelvic and tail. -

Nonnekes Gait Upper Motor Neuron Syndrome Clean
A review of the management of gait impairments in chronic unilateral upper motor neuron lesions Jorik Nonnekes MD PhD1, 2, Nathalie Benda MD PhD2, Hanneke van Duijnhoven MD1, Frits Lem MD2, Noël Keijsers PhD3, Jan Willem K. Louwerens MD PhD4, Allan Pieterse PT PhD1, Bertjo Renzenbrink MD,5 Vivian Weerdesteyn PT PhD,1,3 Jaap Buurke PT PhD,6,7 Alexander C.H. Geurts MD PhD1,2 1Department of Rehabilitation, Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, The Netherlands; 2Department of Rehabilitation, Sint Maartenskliniek, Nijmegen, The Netherlands 3Research Department, Sint Maartenskliniek, Nijmegen, The Netherlands 4Department of Orthopaedics, Sint Maartenskliniek, Nijmegen, The Netherlands 5Rijndam Rehabilitation Center, Rotterdam, The Netherlands 6Roessingh Research and Development, Enschede, the Netherlands 7Biomedical Signals and Systems, MIRA - Institute for Biomedical Technology and Technical Medicine, University of Twente, Enschede, The Netherlands Running title: Gait impairments in supratentorial upper motor neuron syndromes Word count: 3497 Corresponding author Jorik Nonnekes, MD, PhD Radboud University Medical Centre Department of Rehabilitation PO Box 19101, 6500 HB Nijmegen The Netherlands E-mail: [email protected] ABSTRACT Importance: A variety of neurological disorders can damage the corticospinal tract in the supratentorial region of the brain. Gait impairments are common in patients with chronic supratentorial upper motor neuron lesions, and are a source of great disability. Clinical management aimed at improving the gait pattern in these patients is generally perceived as a challenging task, as many possible abnormalities may interact. Moreover, a multitude of treatment options exist – ranging from assistive devices and muscle stretching to pharmacological and surgical interventions – but evidence is inconclusive for most approaches and there is a lack of clear treatment guidelines.