Magnetostatic Modeling for Microfluidic Device Design
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrostatics Vs Magnetostatics Electrostatics Magnetostatics
Electrostatics vs Magnetostatics Electrostatics Magnetostatics Stationary charges ⇒ Constant Electric Field Steady currents ⇒ Constant Magnetic Field Coulomb’s Law Biot-Savart’s Law 1 ̂ ̂ 4 4 (Inverse Square Law) (Inverse Square Law) Electric field is the negative gradient of the Magnetic field is the curl of magnetic vector electric scalar potential. potential. 1 ′ ′ ′ ′ 4 |′| 4 |′| Electric Scalar Potential Magnetic Vector Potential Three Poisson’s equations for solving Poisson’s equation for solving electric scalar magnetic vector potential potential. Discrete 2 Physical Dipole ′′′ Continuous Magnetic Dipole Moment Electric Dipole Moment 1 1 1 3 ∙̂̂ 3 ∙̂̂ 4 4 Electric field cause by an electric dipole Magnetic field cause by a magnetic dipole Torque on an electric dipole Torque on a magnetic dipole ∙ ∙ Electric force on an electric dipole Magnetic force on a magnetic dipole ∙ ∙ Electric Potential Energy Magnetic Potential Energy of an electric dipole of a magnetic dipole Electric Dipole Moment per unit volume Magnetic Dipole Moment per unit volume (Polarisation) (Magnetisation) ∙ Volume Bound Charge Density Volume Bound Current Density ∙ Surface Bound Charge Density Surface Bound Current Density Volume Charge Density Volume Current Density Net , Free , Bound Net , Free , Bound Volume Charge Volume Current Net , Free , Bound Net ,Free , Bound 1 = Electric field = Magnetic field = Electric Displacement = Auxiliary -

Review of Electrostatics and Magenetostatics
Review of electrostatics and magenetostatics January 12, 2016 1 Electrostatics 1.1 Coulomb’s law and the electric field Starting from Coulomb’s law for the force produced by a charge Q at the origin on a charge q at x, qQ F (x) = 2 x^ 4π0 jxj where x^ is a unit vector pointing from Q toward q. We may generalize this to let the source charge Q be at an arbitrary postion x0 by writing the distance between the charges as jx − x0j and the unit vector from Qto q as x − x0 jx − x0j Then Coulomb’s law becomes qQ x − x0 x − x0 F (x) = 2 0 4π0 jx − xij jx − x j Define the electric field as the force per unit charge at any given position, F (x) E (x) ≡ q Q x − x0 = 3 4π0 jx − x0j We think of the electric field as existing at each point in space, so that any charge q placed at x experiences a force qE (x). Since Coulomb’s law is linear in the charges, the electric field for multiple charges is just the sum of the fields from each, n X qi x − xi E (x) = 4π 3 i=1 0 jx − xij Knowing the electric field is equivalent to knowing Coulomb’s law. To formulate the equivalent of Coulomb’s law for a continuous distribution of charge, we introduce the charge density, ρ (x). We can define this as the total charge per unit volume for a volume centered at the position x, in the limit as the volume becomes “small”. -
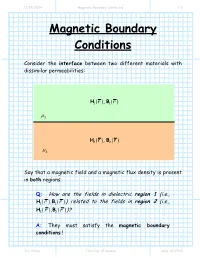
Magnetic Boundary Conditions 1/6
11/28/2004 Magnetic Boundary Conditions 1/6 Magnetic Boundary Conditions Consider the interface between two different materials with dissimilar permeabilities: HB11(r,) (r) µ1 HB22(r,) (r) µ2 Say that a magnetic field and a magnetic flux density is present in both regions. Q: How are the fields in dielectric region 1 (i.e., HB11()rr, ()) related to the fields in region 2 (i.e., HB22()rr, ())? A: They must satisfy the magnetic boundary conditions ! Jim Stiles The Univ. of Kansas Dept. of EECS 11/28/2004 Magnetic Boundary Conditions 2/6 First, let’s write the fields at the interface in terms of their normal (e.g.,Hn ()r ) and tangential (e.g.,Ht (r ) ) vector components: H r = H r + H r H1n ()r 1 ( ) 1t ( ) 1n () ˆan µ 1 H1t (r ) H2t (r ) H2n ()r H2 (r ) = H2t (r ) + H2n ()r µ 2 Our first boundary condition states that the tangential component of the magnetic field is continuous across a boundary. In other words: HH12tb(rr) = tb( ) where rb denotes to any point along the interface (e.g., material boundary). Jim Stiles The Univ. of Kansas Dept. of EECS 11/28/2004 Magnetic Boundary Conditions 3/6 The tangential component of the magnetic field on one side of the material boundary is equal to the tangential component on the other side ! We can likewise consider the magnetic flux densities on the material interface in terms of their normal and tangential components: BHrr= µ B1n ()r 111( ) ( ) ˆan µ 1 B1t (r ) B2t (r ) B2n ()r BH222(rr) = µ ( ) µ2 The second magnetic boundary condition states that the normal vector component of the magnetic flux density is continuous across the material boundary. -

Magnetostatics: Part 1 We Present Magnetostatics in Comparison with Electrostatics
Magnetostatics: Part 1 We present magnetostatics in comparison with electrostatics. Sources of the fields: Electric field E: Coulomb’s law Magnetic field B: Biot-Savart law Forces exerted by the fields: Electric: F = qE Mind the notations, both Magnetic: F = qvB printed and hand‐written Does the magnetic force do any work to the charge? F B, F v Positive charge moving at v B Negative charge moving at v B Steady state: E = vB By measuring the polarity of the induced voltage, we can determine the sign of the moving charge. If the moving charge carriers is in a perfect conductor, then we can have an electric field inside the perfect conductor. Does this contradict what we have learned in electrostatics? Notice that the direction of the magnetic force is the same for both positive and negative charge carriers. Magnetic force on a current carrying wire The magnetic force is in the same direction regardless of the charge carrier sign. If the charge carrier is negative Carrier density Charge of each carrier For a small piece of the wire dl scalar Notice that v // dl A current-carrying wire in an external magnetic field feels the force exerted by the field. If the wire is not fixed, it will be moved by the magnetic force. Some work must be done. Does this contradict what we just said? For a wire from point A to point B, For a wire loop, If B is a constant all along the loop, because Let’s look at a rectangular wire loop in a uniform magnetic field B. -

Modeling of Ferrofluid Passive Cooling System
Excerpt from the Proceedings of the COMSOL Conference 2010 Boston Modeling of Ferrofluid Passive Cooling System Mengfei Yang*,1,2, Robert O’Handley2 and Zhao Fang2,3 1M.I.T., 2Ferro Solutions, Inc, 3Penn. State Univ. *500 Memorial Dr, Cambridge, MA 02139, [email protected] Abstract: The simplicity of a ferrofluid-based was to develop a model that supports results passive cooling system makes it an appealing from experiments conducted on a cylindrical option for devices with limited space or other container of ferrofluid with a heat source and physical constraints. The cooling system sink [Figure 1]. consists of a permanent magnet and a ferrofluid. The experiments involved changing the Ferrofluids are composed of nanoscale volume of the ferrofluid and moving the magnet ferromagnetic particles with a temperature- to different positions outside the ferrofluid dependant magnetization suspended in a liquid container. These experiments tested 1) the effect solvent. The cool, magnetic ferrofluid near the of bringing the heat source and heat sink closer heat sink is attracted toward a magnet positioned together and using less ferrofluid, and 2) the near the heat source, thereby displacing the hot, optimal position for the permanent magnet paramagnetic ferrofluid near the heat source and between the heat source and sink. In the model, setting up convective cooling. This paper temperature-dependent magnetic properties were explores how COMSOL Multiphysics can be incorporated into the force component of the used to model a simple cylinder representation of momentum equation, which was coupled to the such a cooling system. Numerical results from heat transfer module. The model was compared the model displayed the same trends as empirical with experimental results for steady-state data from experiments conducted on the cylinder temperature trends and for appropriate velocity cooling system. -

Computational Study of Triboelectric Generator Design
Rhode Island College Digital Commons @ RIC Honors Projects Overview Honors Projects 2020 Computational Study of Triboelectric Generator Design Giovanni Barbosa Follow this and additional works at: https://digitalcommons.ric.edu/honors_projects COMPUTATIONAL STUDY OF TRIBOELECTRIC GENERATOR DESIGN By Giovannia Barbosa An Honors Project Submitted in Partial Fulfillment of the Requirements for Honors In The Department of Physical Sciences Faculty of Arts and Sciences Rhode Island College 2020 2 Abstract: As the world population increases and technology becomes accessible to more people, there is an increased demand for energy. Researchers are seeking innovative forms of harvesting sustainable energy with minimal environmental impact. The triboelectric effect is a form of contact electrification in which a charge is generated after two materials are separated. In 2012, the triboelectric nanogenerator (TENG) emerged as a new flexible power source which can extract energy from small ambient movements. In this study, we use a software tool known as COMSOL to conduct Finite Element Analysis of Triboelectric Generators (TEGs). One aspect we study is the enhancement of TEG efficiency by the inclusion of high dielectric constant (high-k) materials. We show that the orientation of inclusions matter: high-k dielectric inclusions with a vertical orientation display greater overall TEG performance in comparison to those with a horizontal alignment. A design where a piezoelectric material is included in the triboelectric polymer is also explored. Although -

COMSOL Multiphysics®
COMSOL Multiphysics ® M ODEL L IBRARY V ERSION 3.5a How to contact COMSOL: Germany United Kingdom COMSOL Multiphysics GmbH COMSOL Ltd. Benelux Berliner Str. 4 UH Innovation Centre COMSOL BV D-37073 Göttingen College Lane Röntgenlaan 19 Phone: +49-551-99721-0 Hatfield 2719 DX Zoetermeer Fax: +49-551-99721-29 Hertfordshire AL10 9AB The Netherlands [email protected] Phone:+44-(0)-1707 636020 Phone: +31 (0) 79 363 4230 www.comsol.de Fax: +44-(0)-1707 284746 Fax: +31 (0) 79 361 4212 [email protected] [email protected] Italy www.uk.comsol.com www.comsol.nl COMSOL S.r.l. Via Vittorio Emanuele II, 22 United States Denmark 25122 Brescia COMSOL, Inc. COMSOL A/S Phone: +39-030-3793800 1 New England Executive Park Diplomvej 376 Fax: +39-030-3793899 Suite 350 2800 Kgs. Lyngby [email protected] Burlington, MA 01803 Phone: +45 88 70 82 00 www.it.comsol.com Phone: +1-781-273-3322 Fax: +45 88 70 80 90 Fax: +1-781-273-6603 [email protected] Norway www.comsol.dk COMSOL AS COMSOL, Inc. Søndre gate 7 10850 Wilshire Boulevard Finland NO-7485 Trondheim Suite 800 COMSOL OY Phone: +47 73 84 24 00 Los Angeles, CA 90024 Arabianranta 6 Fax: +47 73 84 24 01 Phone: +1-310-441-4800 FIN-00560 Helsinki [email protected] Fax: +1-310-441-0868 Phone: +358 9 2510 400 www.comsol.no Fax: +358 9 2510 4010 COMSOL, Inc. [email protected] Sweden 744 Cowper Street www.comsol.fi COMSOL AB Palo Alto, CA 94301 Tegnérgatan 23 Phone: +1-650-324-9935 France SE-111 40 Stockholm Fax: +1-650-324-9936 COMSOL France Phone: +46 8 412 95 00 WTC, 5 pl. -

Classical Electromagnetism - Wikipedia, the Free Encyclopedia Page 1 of 6
Classical electromagnetism - Wikipedia, the free encyclopedia Page 1 of 6 Classical electromagnetism From Wikipedia, the free encyclopedia (Redirected from Classical electrodynamics) Classical electromagnetism (or classical electrodynamics ) is a Electromagnetism branch of theoretical physics that studies consequences of the electromagnetic forces between electric charges and currents. It provides an excellent description of electromagnetic phenomena whenever the relevant length scales and field strengths are large enough that quantum mechanical effects are negligible (see quantum electrodynamics). Fundamental physical aspects of classical electrodynamics are presented e.g. by Feynman, Electricity · Magnetism Leighton and Sands, [1] Panofsky and Phillips, [2] and Jackson. [3] Electrostatics Electric charge · Coulomb's law · The theory of electromagnetism was developed over the course of the 19th century, most prominently by James Clerk Maxwell. For Electric field · Electric flux · a detailed historical account, consult Pauli, [4] Whittaker, [5] and Gauss's law · Electric potential · Pais. [6] See also History of optics, History of electromagnetism Electrostatic induction · and Maxwell's equations . Electric dipole moment · Polarization density Ribari č and Šušteršič[7] considered a dozen open questions in the current understanding of classical electrodynamics; to this end Magnetostatics they studied and cited about 240 references from 1903 to 1989. Ampère's law · Electric current · The outstanding problem with classical electrodynamics, as stated Magnetic field · Magnetization · [3] by Jackson, is that we are able to obtain and study relevant Magnetic flux · Biot–Savart law · solutions of its basic equations only in two limiting cases: »... one in which the sources of charges and currents are specified and the Magnetic dipole moment · resulting electromagnetic fields are calculated, and the other in Gauss's law for magnetism which external electromagnetic fields are specified and the Electrodynamics motion of charged particles or currents is calculated.. -

Fields, Units, Magnetostatics
Fields, Units, Magnetostatics European School on Magnetism Laurent Ranno ([email protected]) Institut N´eelCNRS-Universit´eGrenoble Alpes 10 octobre 2017 European School on Magnetism Laurent Ranno ([email protected])Fields, Units, Magnetostatics Motivation Magnetism is around us and magnetic materials are widely used Magnet Attraction (coins, fridge) Contactless Force (hand) Repulsive Force : Levitation Magnetic Energy - Mechanical Energy (Magnetic Gun) Magnetic Energy - Electrical Energy (Induction) Magnetic Liquids A device full of magnetic materials : the Hard Disk drive European School on Magnetism Laurent Ranno ([email protected])Fields, Units, Magnetostatics reminders Flat Disk Rotary Motor Write Head Voice Coil Linear Motor Read Head Discrete Components : Transformer Filter Inductor European School on Magnetism Laurent Ranno ([email protected])Fields, Units, Magnetostatics Magnetostatics How to describe Magnetic Matter ? How Magnetic Materials impact field maps, forces ? How to model them ? Here macroscopic, continous model Next lectures : Atomic magnetism, microscopic details (exchange mechanisms, spin-orbit, crystal field ...) European School on Magnetism Laurent Ranno ([email protected])Fields, Units, Magnetostatics Magnetostatics w/o magnets : Reminder Up to 1820, magnetism and electricity were two subjects not experimentally connected H.C. Oersted experiment (1820 - Copenhagen) European School on Magnetism Laurent Ranno ([email protected])Fields, Units, Magnetostatics Magnetostatics induction field B Looking for a mathematical expression Fields and forces created by an electrical circuit (C1, I) Elementary dB~ induction field created at M ~ ~ µ0I dl^u~ Biot and Savart law (1820) dB(M) = 4πr 2 European School on Magnetism Laurent Ranno ([email protected])Fields, Units, Magnetostatics Magnetostatics : Vocabulary µ I dl~ ^ u~ dB~ (M) = 0 4πr 2 B~ is the magnetic induction field ~ 1 1 B is a long-range vector field ( r 2 becomes r 3 for a closed circuit). -

Educational Electronic Course ”Theory of the Electromagnetic Field” on the Basis of the Program Complex COMSOL Multiphysic
Educational electronic course ”Theory of the electromagnetic field” on the basis of the program complex COMSOL Multiphysics Dudnikov Evgeny, Institute of Control Problem, Moscow, Russia [email protected] Shmelev Vjacheslav, Vladimir State Technical University, Vladimir, Russia [email protected] Walden Bertil, COMSOL AB, Stockholm, Sweden [email protected] Abstract In the submitted paper the educational electronic course «Theory of the electromagnetic field» which uses the basic tools of the program complex COMSOL Multiphysics is considered. A basis of this electronic course is the standard course of lectures «Theory of the electromagnetic field» usually presented to the students who are studying the electrotechnical specialities in the Russian technical universities. The contents of the course includes five sections (General problems of the theory of the electromagnetic field (EMF), the Electrostatic field, the Electric field of a direct current in conducting medium, the Magnetostatic field and the Variable harmonic EMF). We have not include in structure of the course the section “The EMF in moving mediums” because this section usually does not a part of the standard student’s course for these specialities. A great number of exercises and demonstration examples which need to show, how with help of COMSOL Multiphysics it is possible to solve the various problems of the EMF theory, is entered into the course. Among the used tools of COMSOL Multiphysics there are the COMSOL Script, the PDE Modes, the Electromagnetics applications modes of COMSOL Multiphysics nucleus and the Module of electromagnetism. Examples and exercises will allow students to acquire better knowledge from the submitted materials and to receive very important skills of work with modern toolkits for modeling and solving various problems of EMF. -

Lorentz-Violating Electrostatics and Magnetostatics
Publications 10-1-2004 Lorentz-Violating Electrostatics and Magnetostatics Quentin G. Bailey Indiana University - Bloomington, [email protected] V. Alan Kostelecký Indiana University - Bloomington Follow this and additional works at: https://commons.erau.edu/publication Part of the Electromagnetics and Photonics Commons, and the Physics Commons Scholarly Commons Citation Bailey, Q. G., & Kostelecký, V. A. (2004). Lorentz-Violating Electrostatics and Magnetostatics. Physical Review D, 70(7). https://doi.org/10.1103/PhysRevD.70.076006 This Article is brought to you for free and open access by Scholarly Commons. It has been accepted for inclusion in Publications by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. Lorentz-Violating Electrostatics and Magnetostatics Quentin G. Bailey and V. Alan Kosteleck´y Physics Department, Indiana University, Bloomington, IN 47405, U.S.A. (Dated: IUHET 472, July 2004) The static limit of Lorentz-violating electrodynamics in vacuum and in media is investigated. Features of the general solutions include the need for unconventional boundary conditions and the mixing of electrostatic and magnetostatic effects. Explicit solutions are provided for some simple cases. Electromagnetostatics experiments show promise for improving existing sensitivities to parity- odd coefficients for Lorentz violation in the photon sector. I. INTRODUCTION electrodynamics in Minkowski spacetime, coupled to an arbitrary 4-current source. There exists a substantial Since its inception, relativity and its underlying theoretical literature discussing the electrodynamics limit Lorentz symmetry have been intimately linked to clas- of the SME [19], but the stationary limit remains unex- sical electrodynamics. A century after Einstein, high- plored to date. We begin by providing some general in- sensitivity experiments based on electromagnetic phe- formation about this theory, including some aspects as- nomena remain popular as tests of relativity. -

Aharonov-Bohm Detection of Two-Dimensional Magnetostatic Cloaks
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Nazarbayev University Repository PHYSICAL REVIEW B 92, 224414 (2015) Aharonov-Bohm detection of two-dimensional magnetostatic cloaks Constantinos A. Valagiannopoulos Department of Physics, School of Science and Technology, Nazarbayev University, 53 Qabanbay Batyr Avenue, Astana KZ-010000, Kazakhstan Amir Nader Askarpour Department of Electrical Engineering, Amirkabir University of Technology, 424 Hafez Avenue, Tehran 15875-4413, Iran Andrea Alu` Department of Electrical Engineering, University of Texas at Austin, 1616 Guadalupe Street, Austin, Texas 78701, USA (Received 29 September 2015; revised manuscript received 16 November 2015; published 10 December 2015) Two-dimensional magnetostatic cloaks, even when perfectly designed to mitigate the magnetic field disturbance of a scatterer, may be still detectable with Aharonov-Bohm (AB) measurements, and therefore may affect quantum interactions and experiments with elongated objects. We explore a multilayered cylindrical cloak whose permeability profile is tailored to nullify the magnetic-flux perturbation of the system, neutralizing its effect on AB measurements, and simultaneously optimally suppress the overall scattering. In this way, our improved magnetostatic cloak combines substantial mitigation of the magnetostatic scattering response with zero detectability by AB experiments. DOI: 10.1103/PhysRevB.92.224414 PACS number(s): 41.20.Gz, 03.65.Ta, 94.05.Pt, 85.25.Dq I. INTRODUCTION approach, for which no anisotropic or inhomogeneous media are necessary. An alternative experimental demonstration [12] Making an electromagnetic object of arbitrary shape and was reported in the quasistatic regime, and a magnetostatic texture invisible to certain portions of the frequency spec- carpet cloak [13] to hide objects over a superconducting plane trum is one of the most intriguing possibilities offered by is based again on transformation optics.